filmov
tv
7. Kevin Ahern's Biochemistry - Protein Purification II

Показать описание
Protein Purification/Characterization II
1. Isoelectric focusing separates molecules on the basis of their pI. It is performed in tubes containing polyelectrolytes that migrate to specific points in the tube in the presence of an electric field. This creates a pH gradient from one end of the tube to the other. If proteins are added to the tube as the gradient is getting established, they will migrate to the point in the tube where the pH corresponds to their pI and they will migrate nor further.
2. 2D gel electrophoresis is a tool for proteomics that combines the techniques of isoelectric focusing with SDS-PAGE. In this method, proteins are separated according to their pI by isoelectric focusing. Then the tube from the isoelectric focusing is applied to the top of an SDS-PAGE gel and the proteins are separated by size. The result is a two dimensional separation of virtually every protein in the cell.
3. During purification of proteins it is important to follow the purification process. At each step of the purification, a small sample of the protein extract is taken and the total amount of protein, and the amount of activity of the desired protein are measured. The specific activity of the protein in the tube is the amount of activity divided by the total mass of protein. The yield of the desired protein at any point in the purification process is the number of units of the desired protein at that point dividied by the number of units that one started with. The purification level at any point is the specific activity at that point divided by the specific activity one started with.
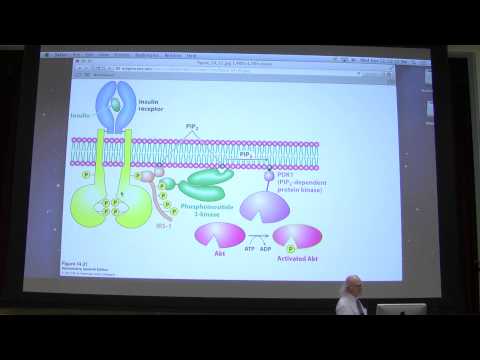
4. MALDI-TOF is a method for determining the molecular weight of relatively large molecules, such as polypeptides. It works by introduction of a sample into the evacuated chamber of a MALDI-TOF instrument. A laser is aimed at the sample, which volatilizes and ionizes, creating a charged molecule. Application of an electric field "drives" the charged molecule towards a detector. By measuring the time it takes the charged molecule to get to the detector, one can determine its mass by the fact that heavier molecules will have a lower charge to mass ratio than lighter molecules and will thus travel slower.
5. Most methods for identifying proteins aren't very good for working on intact proteins. Methods to specifically break polypeptides down to smaller pieces are thus desired. They include cyanogen bromide, trypsin, chymotrypsin, and carboxypeptidase.
6. DNA sequencing techniques are so sophisticated now that it is easier to sequence a million bases of DNA than to do a single Edman degradation. Thus, if the DNA sequence coding for a protein is known, that is the method of choice.
7. Immunological techniques aid in identifying specific proteins. Antibodies (immune system proteins) recognize and bind to specific structures. The structures antibodies bind to are called antigens. Usually antibodies are targeted against specific protein structures. Since proteins differ from each other in their structure, molecules (like antibodies) that bind to specific structures will bind to specific proteins.
8. Antibodies are very useful in that they can be linked to fluorescent dyes, gold particles, or enzymes to help one visualize where an antibody has bound. They are useful in binding to specific cellular structures (in situ hydridization), to identify where within a cell or within a tissue a particular protein is located.
9. One technique that employs antibodies is western blotting. In this technique, a mixture of proteins is separated by SDS-PAGE. The proteins in the gel are transferred directly to a membrane, which is then treated with an antibody specific for one of the proteins. The membrane is washed to release unbound antibody and then the antibody-protein complex is visualized. This can be by a fluorescent technique or more commonly by an enzyme linked to the antibody as above. In any case, the protein of interest is identified in this way.
Комментарии
 0:46:36
0:46:36
 0:43:55
0:43:55
 0:49:49
0:49:49
 0:48:57
0:48:57
 0:23:43
0:23:43
 1:05:25
1:05:25
 0:47:08
0:47:08
 0:47:21
0:47:21
 0:48:30
0:48:30
 0:48:45
0:48:45
 0:50:30
0:50:30
 0:49:17
0:49:17
 0:48:58
0:48:58
 0:47:45
0:47:45
 0:50:49
0:50:49
 0:50:02
0:50:02
 0:50:46
0:50:46
 0:52:45
0:52:45
 1:01:51
1:01:51
 0:47:41
0:47:41
 0:48:34
0:48:34
 0:50:22
0:50:22
 1:07:01
1:07:01
 0:51:46
0:51:46