filmov
tv
Synthesis Workshop: High-valent Bismuth Redox Catalysis with Dr. Oriol Planas (Episode 36)

Показать описание
In this Research Spotlight episode, we are joined by Dr. Oriol Planas, who takes us through his work in the area of bismuth catalysis!
Parent references:
Science 2020, 367, 313-317.
J. Am. Chem. Soc. 2020, 142, 11382-11387.
For footnotes, see references.
Other references (in order of appearance):
For a highlight on the benign properties of bismuth, see Nat. Chem. 2010, 2, 336.
For Bismuth in organic chemistry, see: Bismuth-Mediated Organic Reactions. (Springer, Heidelberg, 2012).
For main group redox processes, see: ChemCatChem, 2018, 10, 4213; Science 2019, 363, 479.
For pioneering work of Suzuki and co-workers on sulfone-based bismacycles, see: J. Chem. Soc., Perkin Trans. 1, 1992, 1593.
For a catalytic fluorination of organoboron compounds through SET process, see: J. Am. Chem. Soc. 2013, 135, 14012-14015.
For mechanistic considerations of negative entropies, see: Chem. Sci. 2012, 3, 72.; Inorg. Chem. 1998, 37, 3975.
For transmetallation studies to Bi(III) centers, see: Organometallics 2007, 26, 6864; Nat. Chem. 2020, 12, 260.
For pioneer studies on Bi-mediated C-O bond formation, see: Chem. Lett. 2007, 36, 928.
For studies on effects of molecular sieves in catalysis, see: J. Org. Chem. 2006, 71, 1861; Chem. Rev. 2019, 119, 12491.
Parent references:
Science 2020, 367, 313-317.
J. Am. Chem. Soc. 2020, 142, 11382-11387.
For footnotes, see references.
Other references (in order of appearance):
For a highlight on the benign properties of bismuth, see Nat. Chem. 2010, 2, 336.
For Bismuth in organic chemistry, see: Bismuth-Mediated Organic Reactions. (Springer, Heidelberg, 2012).
For main group redox processes, see: ChemCatChem, 2018, 10, 4213; Science 2019, 363, 479.
For pioneering work of Suzuki and co-workers on sulfone-based bismacycles, see: J. Chem. Soc., Perkin Trans. 1, 1992, 1593.
For a catalytic fluorination of organoboron compounds through SET process, see: J. Am. Chem. Soc. 2013, 135, 14012-14015.
For mechanistic considerations of negative entropies, see: Chem. Sci. 2012, 3, 72.; Inorg. Chem. 1998, 37, 3975.
For transmetallation studies to Bi(III) centers, see: Organometallics 2007, 26, 6864; Nat. Chem. 2020, 12, 260.
For pioneer studies on Bi-mediated C-O bond formation, see: Chem. Lett. 2007, 36, 928.
For studies on effects of molecular sieves in catalysis, see: J. Org. Chem. 2006, 71, 1861; Chem. Rev. 2019, 119, 12491.
Synthesis Workshop: High-valent Bismuth Redox Catalysis with Dr. Oriol Planas (Episode 36)
Synthesis Workshop: Selective Dearomatization of Phenols with Prof. Sarah Wengryniuk (Episode 71)
Synthesis Workshop: Synthesis of Optically Active 7-Membered Rings with Michele Garbo (Episode 25)
Synthesis Workshop: (-)-PF-1018 Total Synthesis (Episode 16)
Synthesis Workshop: Asymmetric C–P Coupling with Dr. Anirban Mondal (Episode 99)
Synthesis Workshop: Total Synthesis of (±)-Leonuketal with Dr. Phillip Grant (Episode 37)
Homobenzylic Oxygenation via Dual Organic Photoredox and Cobalt Catalysis with Dr. Joshua McManus
Josep Cornella — Talented 12 Class of 2020
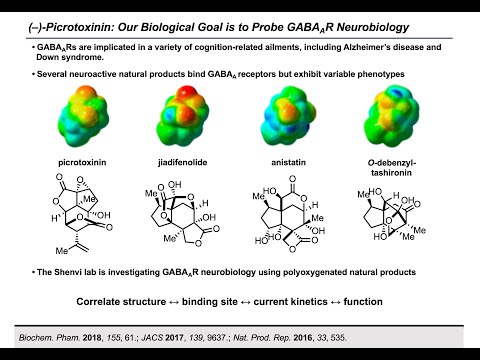
Synthesis Workshop: Synthesis of Picrotoxinin and Picrotin with Dr. Steven Crossley (Episode 27)
Obtain Bismuth Oxyiodide Microspheres Highly Functional: Photocatalytic Processes l Protocol Preview
Synthesis Workshop: Total Synthesis of Chilocorine C with Vlad Lisnyak (Episode 24)
Redox Corporate Offices
Synthesis Workshop: Syntheses of Macfarlandin C + Dendrillolide A with Dr. Tyler Allred (Episode 40)
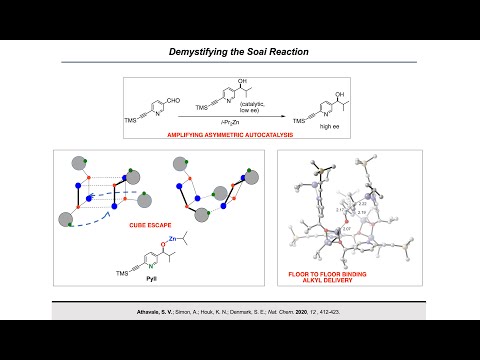
Synthesis Workshop: Demystifying the Soai Reaction with Dr. Soumitra Athavale (Episode 47)
Synthesis Workshop: The Schlenk Line Survival Guide with Dr. Andryj Borys (Episode 45)
Synthesis Workshop: Total Syntheses of GB22, GB13, and Himgaline with Eleanor Landwehr (Episode 103)
Short Video: Bismuth Can Act Like a Transition-Metal Catalyst
Synthesis Workshop: The Kulinkovich Reaction (Episode 42)
Bismuth High Impact Costs Estimate : Passive deactivation of radionuclides - Experiment series
CCHF VS 11.4 - Prof. Jessica Hoover | Oxidative Decarboxylative Arylation Reactions of C–H bonds
Synthesis of Bismuth(III)-nitrate
Bismuth Quantum Optics! Next level nano-photonics.
Prof. Alois Fürstner introduces Science of Synthesis at ESOC 2019 in Vienna
Oxidation States and Redox Properties of Transition Metals | OpenStax Chemistry 2e 19.1
Комментарии
 0:17:35
0:17:35
 0:21:07
0:21:07
 0:13:46
0:13:46
 0:05:46
0:05:46
 0:21:58
0:21:58
 0:09:53
0:09:53
 0:12:31
0:12:31
 0:15:59
0:15:59
 0:23:45
0:23:45
 0:02:01
0:02:01
 0:17:31
0:17:31
 0:02:36
0:02:36
 0:19:36
0:19:36
 0:30:12
0:30:12
 0:13:59
0:13:59
 0:16:04
0:16:04
 0:01:00
0:01:00
 0:08:49
0:08:49
 0:06:15
0:06:15
 0:22:46
0:22:46
 0:02:39
0:02:39
 0:01:01
0:01:01
 0:09:32
0:09:32
 0:05:01
0:05:01