filmov
tv
Structure of Atom - Full Chapter Explanation and NCERT Solutions | Class 11 Chemistry Chapter 2

Показать описание
==========================
✅ In this video,
✔️ Class: 11th
✔️ Subject: Chemistry
✔️ Chapter: Structure of Atom (Chapter 2)
✔️ Topic Name: Structure of Atom - Full Chapter Explanation and NCERT Solutions | Class 11 Chemistry Chapter 2
✔️ Topics Covered In This Video : This video provides Full Chapter explanation and NCERT Solutions of chapter 2 Structure of Atom from class 11 Chemistry. Be prepared for upcoming exams such as IIT JEE, NEET, and other Class 11 Chemistry exams. Learn all this and more with Anurag Sir in this detailed lecture.
=========================
00:00 Introduction: Structure of Atom
03:05 Dalton's Atomic model (1808)
06:24 A/c to This Theory
13:19 Discovery of an electron OR Cathode Ray Experiment
26:50 Properties of Cathode Rays
52:31 Subatomic Particles
01:00:38 Properties of Canal Rays
01:26:44 Millikan's Oil Drop Method (for determination of charge on electron)
01:33:59 Millikan's Oil Drop Method
01:38:14 Thomson and Rutherford Atomic Model
01:43:14 Rutherford & particle scattering experiment (1911)
02:18:16 Isotopes
02:21:40 Isotopes of Carbon
02:23:19 Example of Isotopes
02:26:24 Isobars
02:28:10 Isotones
02:29:56 Example of Isotones
02:31:00 Isoelectronics
02:33:06 Isodiaphers
02:36:11 Complete the following table - 1
02:36:35 Complete the following table - 2
02:47:49 Bohr's Model
03:01:51 Bohr's Atomic Model (1913)
03:11:54 Calculation of Radius of Orbit
03:23:41 Calculation for Velocity
03:27:51 Derivation for Energy of e
03:45:34 Bohr's Model Part 2
03:48:00 Quantization of Electronic Energy
03:52:18 Usefulness of Bohr's Model
04:06:52 Limitations of Bohr's Model of Atom
04:14:06 Wave Nature of EM Radiation
04:19:52 Wave Nature of EM Radiation (James maxwell- 1870) (EM Wave theory)
04:31:13 Electromagnetic Spectrum
04:51:11 Question 1 & 2: Important Questions of Atomic Structure: Structure of Atom
04:57:24 Particle Nature of EM Radiation (Planck's Quantum Theory)
04:59:29 Particle Nature of EM Radiation (energy travel in form of packet)
05:01:17 Partial Nature of EM Radiation
05:13:27 Question & Solutions: Numericals: Structure of Atom
05:17:07 Photo Electric Effect
05:18:33 Photo Electric Effect (1887 Hartz)
05:20:29 In P.E.E
05:28:13 Important Concept
05:29:54 Einstein Photoelectric Equation.
05:34:20 Question & Solutions: Numericals: Structure of Atom
05:40:34 Atomic Spectrum
06:06:24 Line spectrum of Hydrogen
06:27:55 Points to Remember
06:31:16 Quantum Models of Atom
06:34:25 Derivation (the wavelength of wave associated with any material particle)
06:38:46 Derivation
06:50:22 Heisenberg's Uncertainty Principle
06:53:47 Heisenberg's Uncertainty Principle (1927)
07:27:22 Schrödinger Equation
07:41:20 Quantum Mechanical Model of Atom
07:50:08 Pauli Exclusion Principle
08:08:36 Hund's Rule of Maximum Multiplicity
08:23:35 Stability of Completely filled and Half Filled Subshells
08:34:21 Shapes of Atomic Orbitals
08:36:11 Shapes of s -Orbitals
08:48:25 Shapes of p -Orbitals
08:54:32 Shapes of d -Orbitals
09:00:49 Calculation of nodes
09:07:26 Question 1 to 35: NCERT Exercises of Atomic Structure: Structure of Atom
11:15:26 Website Overview
==============================
👉 Contact us 🤑🤑
#class11chemistry #magnetbrains #ncertsolutions #structureofatomclass11
structure of atom class 11 pdf
structure of atom class 11 notes
structure of atom class 11 notes self study
structure of atom class 11 notes pdf
structure of atom class 11 notes pdf for neet
structure of atom class 11 ncert
structure of atom pdf
structure of atom class 11 handwritten notes
✅ In this video,
✔️ Class: 11th
✔️ Subject: Chemistry
✔️ Chapter: Structure of Atom (Chapter 2)
✔️ Topic Name: Structure of Atom - Full Chapter Explanation and NCERT Solutions | Class 11 Chemistry Chapter 2
✔️ Topics Covered In This Video : This video provides Full Chapter explanation and NCERT Solutions of chapter 2 Structure of Atom from class 11 Chemistry. Be prepared for upcoming exams such as IIT JEE, NEET, and other Class 11 Chemistry exams. Learn all this and more with Anurag Sir in this detailed lecture.
=========================
00:00 Introduction: Structure of Atom
03:05 Dalton's Atomic model (1808)
06:24 A/c to This Theory
13:19 Discovery of an electron OR Cathode Ray Experiment
26:50 Properties of Cathode Rays
52:31 Subatomic Particles
01:00:38 Properties of Canal Rays
01:26:44 Millikan's Oil Drop Method (for determination of charge on electron)
01:33:59 Millikan's Oil Drop Method
01:38:14 Thomson and Rutherford Atomic Model
01:43:14 Rutherford & particle scattering experiment (1911)
02:18:16 Isotopes
02:21:40 Isotopes of Carbon
02:23:19 Example of Isotopes
02:26:24 Isobars
02:28:10 Isotones
02:29:56 Example of Isotones
02:31:00 Isoelectronics
02:33:06 Isodiaphers
02:36:11 Complete the following table - 1
02:36:35 Complete the following table - 2
02:47:49 Bohr's Model
03:01:51 Bohr's Atomic Model (1913)
03:11:54 Calculation of Radius of Orbit
03:23:41 Calculation for Velocity
03:27:51 Derivation for Energy of e
03:45:34 Bohr's Model Part 2
03:48:00 Quantization of Electronic Energy
03:52:18 Usefulness of Bohr's Model
04:06:52 Limitations of Bohr's Model of Atom
04:14:06 Wave Nature of EM Radiation
04:19:52 Wave Nature of EM Radiation (James maxwell- 1870) (EM Wave theory)
04:31:13 Electromagnetic Spectrum
04:51:11 Question 1 & 2: Important Questions of Atomic Structure: Structure of Atom
04:57:24 Particle Nature of EM Radiation (Planck's Quantum Theory)
04:59:29 Particle Nature of EM Radiation (energy travel in form of packet)
05:01:17 Partial Nature of EM Radiation
05:13:27 Question & Solutions: Numericals: Structure of Atom
05:17:07 Photo Electric Effect
05:18:33 Photo Electric Effect (1887 Hartz)
05:20:29 In P.E.E
05:28:13 Important Concept
05:29:54 Einstein Photoelectric Equation.
05:34:20 Question & Solutions: Numericals: Structure of Atom
05:40:34 Atomic Spectrum
06:06:24 Line spectrum of Hydrogen
06:27:55 Points to Remember
06:31:16 Quantum Models of Atom
06:34:25 Derivation (the wavelength of wave associated with any material particle)
06:38:46 Derivation
06:50:22 Heisenberg's Uncertainty Principle
06:53:47 Heisenberg's Uncertainty Principle (1927)
07:27:22 Schrödinger Equation
07:41:20 Quantum Mechanical Model of Atom
07:50:08 Pauli Exclusion Principle
08:08:36 Hund's Rule of Maximum Multiplicity
08:23:35 Stability of Completely filled and Half Filled Subshells
08:34:21 Shapes of Atomic Orbitals
08:36:11 Shapes of s -Orbitals
08:48:25 Shapes of p -Orbitals
08:54:32 Shapes of d -Orbitals
09:00:49 Calculation of nodes
09:07:26 Question 1 to 35: NCERT Exercises of Atomic Structure: Structure of Atom
11:15:26 Website Overview
==============================
👉 Contact us 🤑🤑
#class11chemistry #magnetbrains #ncertsolutions #structureofatomclass11
structure of atom class 11 pdf
structure of atom class 11 notes
structure of atom class 11 notes self study
structure of atom class 11 notes pdf
structure of atom class 11 notes pdf for neet
structure of atom class 11 ncert
structure of atom pdf
structure of atom class 11 handwritten notes
Комментарии
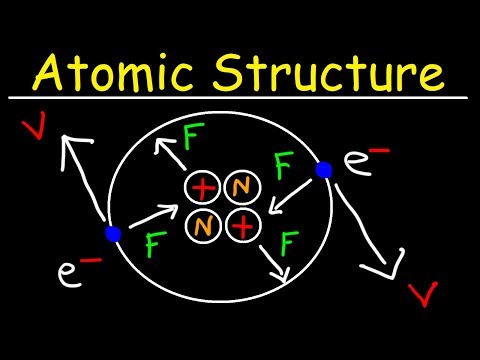 0:11:45
0:11:45
 0:30:31
0:30:31
 1:04:43
1:04:43
 1:57:06
1:57:06
 0:00:12
0:00:12
 0:09:49
0:09:49
 1:29:48
1:29:48
 5:44:28
5:44:28
 0:45:43
0:45:43
 0:59:50
0:59:50
 0:01:44
0:01:44
 3:21:20
3:21:20
 5:13:38
5:13:38
 3:11:55
3:11:55
 1:04:33
1:04:33
 7:19:15
7:19:15
 0:02:02
0:02:02
 1:08:29
1:08:29
 0:04:06
0:04:06
 4:08:34
4:08:34
 1:50:52
1:50:52
 0:36:08
0:36:08
 0:02:20
0:02:20
 3:48:50
3:48:50