filmov
tv
10 Preparation Methods of Amines | Synthesis of Amines | Organic Chemistry

Показать описание
Link to buy the following products:
In this video, we will explain 10 different preparation methods of amines (synthesis of Amines). There are three types of Amines: Primary, Secondary and Tertiary.
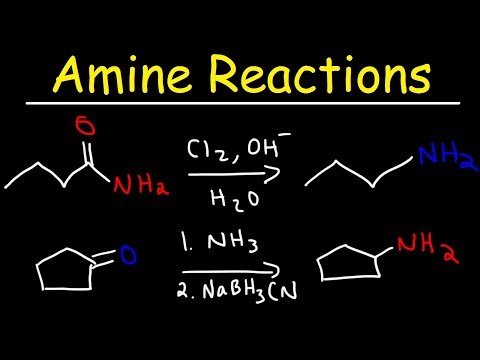
Nucleophilic substitution of haloalkanes:
Primary amines can be synthesized by alkylation of ammonia. A large excess of ammonia is used if the primary amine is the desired product. Haloalkanes react with amines to give a corresponding alkyl-substituted amine, with the release of a halogen acid. Such reactions, which are most useful for alkyl iodides and bromides, are rarely employed because the degree of alkylation is difficult to control. If the reacting amine is tertiary, a quaternary ammonium cation results. Many quaternary ammonium salts can be prepared by this route with diverse R groups and many halide and pseudohalide anions.
When primary amines are heated with halogenoalkanes, a complicated series of reactions occurs, giving a mixture of products – probably one of the most confusing sets of reactions you will meet at this level. The products of the reactions include secondary and tertiary amines and their salts, and quaternary ammonium salts.
Making secondary amines and their salts:
In the first stage of the reaction, you get the salt of a secondary amine formed. For example if you started with ethylamine and bromoethane, you would get diethylammonium bromide
In the presence of excess ethylamine in the mixture, there is the possibility of a reversible reaction. The ethylamine removes a hydrogen from the diethylammonium ion to give free diethylamine – a secondary amine.
Making tertiary amines and their salts:
But it doesn’t stop here! The diethylamine also reacts with bromoethane – in the same two stages as before. This is where the reaction would start if you reacted a secondary amine with a halogenoalkane.
In the first stage, you get triethylammonium bromide.
There is again the possibility of a reversible reaction between this salt and excess ethylamine in the mixture.
The ethylamine removes a hydrogen ion from the triethylammonium ion to leave a tertiary amine – triethylamine.
Preparation of Primary Amines
Although direct alkylation of ammonia (large excess) by alkyl halides leads to 1º-amines, alternative procedures are preferred in many cases. These methods require two steps, but they provide pure product, usually in good yield. The general strategy is to first form a carbon-nitrogen bond by reacting a nitrogen nucleophile with a carbon electrophile. The following table lists several general examples of this strategy in the rough order of decreasing nucleophilicity of the nitrogen reagent. In the second step, extraneous nitrogen substituents that may have facilitated this bonding are removed to give the amine product.
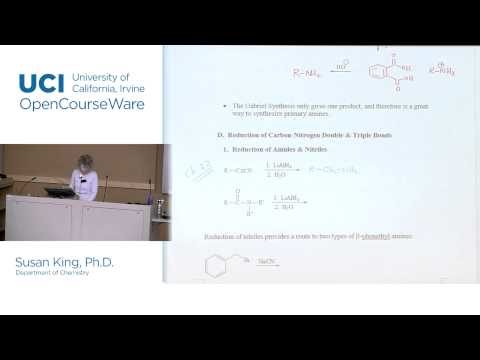
Preparation of Secondary and Tertiary Amines
Of the six methods described above, three are suitable for the preparation of 2º and/or 3º-amines. These are
1. Alkylation of the sulfonamide derivative of a 1º-amine. Gives 2º-amines.
2. Reduction of alkyl imines and dialkyl iminium salts. Gives 2º & 3º-amines.
3. Reduction of amide derivatives of 1º & 2º-amines. Gives 2º & 3º-amines.
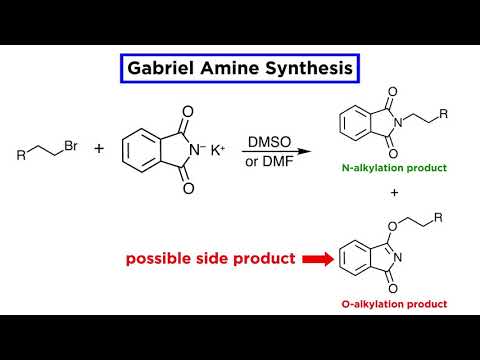
Examples showing the application of these methods to the preparation of specific amines are shown in the following diagram. The sulfonamide procedure used in the first example is similar in concept to the phthalimide example #2 presented in the previous diagram. In both cases the acidity of the nitrogen reactant (ammonia or amine) is greatly enhanced by conversion to an imide or sulfonamide derivative. The nucleophilic conjugate base of this acidic nitrogen species is then prepared by treatment with sodium or potassium hydroxide, and this undergoes an SN2 reaction with a 1º or 2º-alkyl halide. Finally, the activating group is removed by hydrolysis (phthalimide) or reductive cleavage (sulfonamide) to give the desired amine. The phthalimide method is only useful for preparing 1º-amines, whereas the sulfonamide procedure may be used to make either 1º or 2º-amines.
#PreparationofAmines
#10Methods
#PreparationMethodsofAmines
#SynthesisofAmines
#PrimaryAmine
#SecondaryAmine
#TertiaryAmine
#OrganicChemistry
#Alcohol
#Acetal
#Alkene
In this video, we will explain 10 different preparation methods of amines (synthesis of Amines). There are three types of Amines: Primary, Secondary and Tertiary.
Nucleophilic substitution of haloalkanes:
Primary amines can be synthesized by alkylation of ammonia. A large excess of ammonia is used if the primary amine is the desired product. Haloalkanes react with amines to give a corresponding alkyl-substituted amine, with the release of a halogen acid. Such reactions, which are most useful for alkyl iodides and bromides, are rarely employed because the degree of alkylation is difficult to control. If the reacting amine is tertiary, a quaternary ammonium cation results. Many quaternary ammonium salts can be prepared by this route with diverse R groups and many halide and pseudohalide anions.
When primary amines are heated with halogenoalkanes, a complicated series of reactions occurs, giving a mixture of products – probably one of the most confusing sets of reactions you will meet at this level. The products of the reactions include secondary and tertiary amines and their salts, and quaternary ammonium salts.
Making secondary amines and their salts:
In the first stage of the reaction, you get the salt of a secondary amine formed. For example if you started with ethylamine and bromoethane, you would get diethylammonium bromide
In the presence of excess ethylamine in the mixture, there is the possibility of a reversible reaction. The ethylamine removes a hydrogen from the diethylammonium ion to give free diethylamine – a secondary amine.
Making tertiary amines and their salts:
But it doesn’t stop here! The diethylamine also reacts with bromoethane – in the same two stages as before. This is where the reaction would start if you reacted a secondary amine with a halogenoalkane.
In the first stage, you get triethylammonium bromide.
There is again the possibility of a reversible reaction between this salt and excess ethylamine in the mixture.
The ethylamine removes a hydrogen ion from the triethylammonium ion to leave a tertiary amine – triethylamine.
Preparation of Primary Amines
Although direct alkylation of ammonia (large excess) by alkyl halides leads to 1º-amines, alternative procedures are preferred in many cases. These methods require two steps, but they provide pure product, usually in good yield. The general strategy is to first form a carbon-nitrogen bond by reacting a nitrogen nucleophile with a carbon electrophile. The following table lists several general examples of this strategy in the rough order of decreasing nucleophilicity of the nitrogen reagent. In the second step, extraneous nitrogen substituents that may have facilitated this bonding are removed to give the amine product.
Preparation of Secondary and Tertiary Amines
Of the six methods described above, three are suitable for the preparation of 2º and/or 3º-amines. These are
1. Alkylation of the sulfonamide derivative of a 1º-amine. Gives 2º-amines.
2. Reduction of alkyl imines and dialkyl iminium salts. Gives 2º & 3º-amines.
3. Reduction of amide derivatives of 1º & 2º-amines. Gives 2º & 3º-amines.
Examples showing the application of these methods to the preparation of specific amines are shown in the following diagram. The sulfonamide procedure used in the first example is similar in concept to the phthalimide example #2 presented in the previous diagram. In both cases the acidity of the nitrogen reactant (ammonia or amine) is greatly enhanced by conversion to an imide or sulfonamide derivative. The nucleophilic conjugate base of this acidic nitrogen species is then prepared by treatment with sodium or potassium hydroxide, and this undergoes an SN2 reaction with a 1º or 2º-alkyl halide. Finally, the activating group is removed by hydrolysis (phthalimide) or reductive cleavage (sulfonamide) to give the desired amine. The phthalimide method is only useful for preparing 1º-amines, whereas the sulfonamide procedure may be used to make either 1º or 2º-amines.
#PreparationofAmines
#10Methods
#PreparationMethodsofAmines
#SynthesisofAmines
#PrimaryAmine
#SecondaryAmine
#TertiaryAmine
#OrganicChemistry
#Alcohol
#Acetal
#Alkene
Комментарии
 0:10:43
0:10:43
 0:12:32
0:12:32
 0:32:40
0:32:40
 0:29:51
0:29:51
 0:22:50
0:22:50
 0:48:43
0:48:43
 0:09:17
0:09:17
 0:00:12
0:00:12
 1:24:24
1:24:24
 0:06:39
0:06:39
 0:21:55
0:21:55
 0:19:46
0:19:46
 0:03:47
0:03:47
 0:00:19
0:00:19
 0:46:38
0:46:38
 0:04:00
0:04:00
 0:00:24
0:00:24
 0:07:53
0:07:53
 0:00:22
0:00:22
 0:01:00
0:01:00
 0:06:44
0:06:44
 0:01:00
0:01:00
 0:10:52
0:10:52
 0:07:45
0:07:45