filmov
tv
Aldehydes to Secondary Alcohols, Part 2: Organoboron Compounds

Показать описание
This video is about the use of organoboron compounds as nucleophiles. Boron is only slightly less electronegative than carbon, and organoboranes are weak Lewis acids that are not readily hydrolyzed by water. Simple alkylboranes react, however, with elemental oxygen, and must therefore be kept under some inert gas.
Like polar organometallics, organoboranes bind strongly to the oxygen atom of carbonyl groups. Nevertheless, no transfer of simple alkyl groups to the activated carbonyl carbon atom is usually observed. Instead, upon treatment with alkylboranes aldehydes are reduced to primary alcohols. This reduction can proceed by an intramolecular hydride transfer, or by reaction of the aldehyde with a borohydride, the latter being formed by thermal dealkylation of the alkylborane.
Thus, simple alkylboranes cannot be used directly as carbon nucleophiles for the nucleophilic alkylation of aldehydes or ketones. This is, though, not the case for allylboranes.
The scope of this reaction can, however, be improved by some catalysts. Another strategy to enhance the reactivity of alkylboranes is transmetallation with for instance diethylzinc.
Further recent examples:
J. Org. Chem. 2017, 82, 3334-3340 (Et2Zn); 2013, 78, 3592-3615 (propargylation); 2007, 72, 4102-4107 (Pd); 2005, 70, 7408-7417 (Et2Zn, asym. vinylation); 2004, 69, 2532-2543 (Et2Zn, asym.); Org. Lett. 2012, 14, 3962-3965 (Et2Zn); 2009, 11, 4410-4413 (Ni); 2005, 7, 4153-4155 (Pd-CHCl3); 1729-1732 (asym. vinylation); 2004, 6, 2701-2704 (asym. allylation); 2002, 4, 1491-1493 (vinylation); Chem. Commun. 2005, 4484-4486 (Rh); 2512-2514 (Et2Zn, asym.); 1459-1461 (Ni); J. Am. Chem. Soc. 2007, 129, 16119-16125 (Et2Zn, asym. vinylation);
2002, 124, 14850-14851 (Et2Zn, asym.); Beilstein J. Org. Chem. 2009, 5, 56 (continuous); Tetrahedron Lett. 2001, 42, 6957-6960 (Rh).
Like polar organometallics, organoboranes bind strongly to the oxygen atom of carbonyl groups. Nevertheless, no transfer of simple alkyl groups to the activated carbonyl carbon atom is usually observed. Instead, upon treatment with alkylboranes aldehydes are reduced to primary alcohols. This reduction can proceed by an intramolecular hydride transfer, or by reaction of the aldehyde with a borohydride, the latter being formed by thermal dealkylation of the alkylborane.
Thus, simple alkylboranes cannot be used directly as carbon nucleophiles for the nucleophilic alkylation of aldehydes or ketones. This is, though, not the case for allylboranes.
The scope of this reaction can, however, be improved by some catalysts. Another strategy to enhance the reactivity of alkylboranes is transmetallation with for instance diethylzinc.
Further recent examples:
J. Org. Chem. 2017, 82, 3334-3340 (Et2Zn); 2013, 78, 3592-3615 (propargylation); 2007, 72, 4102-4107 (Pd); 2005, 70, 7408-7417 (Et2Zn, asym. vinylation); 2004, 69, 2532-2543 (Et2Zn, asym.); Org. Lett. 2012, 14, 3962-3965 (Et2Zn); 2009, 11, 4410-4413 (Ni); 2005, 7, 4153-4155 (Pd-CHCl3); 1729-1732 (asym. vinylation); 2004, 6, 2701-2704 (asym. allylation); 2002, 4, 1491-1493 (vinylation); Chem. Commun. 2005, 4484-4486 (Rh); 2512-2514 (Et2Zn, asym.); 1459-1461 (Ni); J. Am. Chem. Soc. 2007, 129, 16119-16125 (Et2Zn, asym. vinylation);
2002, 124, 14850-14851 (Et2Zn, asym.); Beilstein J. Org. Chem. 2009, 5, 56 (continuous); Tetrahedron Lett. 2001, 42, 6957-6960 (Rh).
 0:09:56
0:09:56
 0:09:33
0:09:33
 0:09:51
0:09:51
 0:04:34
0:04:34
 0:09:22
0:09:22
 0:08:59
0:08:59
 0:12:28
0:12:28
 0:06:24
0:06:24
 0:07:34
0:07:34
 0:06:47
0:06:47
 0:01:43
0:01:43
 0:07:30
0:07:30
 0:03:14
0:03:14
 0:15:52
0:15:52
 0:07:47
0:07:47
 0:07:40
0:07:40
 0:11:21
0:11:21
 0:06:28
0:06:28
 0:11:44
0:11:44
 0:05:36
0:05:36
 0:05:24
0:05:24
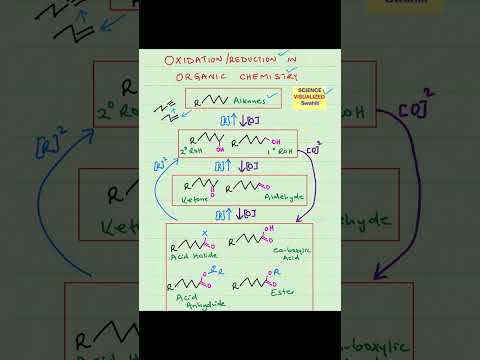 0:00:59
0:00:59
 0:00:15
0:00:15
 0:19:55
0:19:55