filmov
tv
The Chemistry of Fireworks Pollution and Its Environmental Impacts
Показать описание
Across the globe, celebrating important events and holidays will never be complete without having a fireworks display. With the magnificent burst of colors in the night sky and loud explosive noise, fireworks make us feel happy, enjoy, and cherish each celebration. But behind these lights comes the dark side of it. In this video let us zoom in on how fireworks impact our health and environment.
Its development dates back to the second century B.C. Bamboo stalks are considered the earliest natural firecrackers used to ward off evil spirits in ancient Liuyang, China. As a result of the hollow air pockets heating up, they explode when tossed into a fire.
In the 13th century, fireworks began to be recognized and manufactured in Europe by Italians. They were used to celebrate religious festivals, entertain and enchant the public, and illuminate the castles.
The composition inside the firework contains five (4) vital ingredients: oxidizing agent or oxidizers, fuel or reducing agent, coloring agents, and binders.
THE WORKING MECHANISM OF FIREWORKS
Fireworks are stored in a tube called mortar tube or launch tube which helps the aerial shell to sent up into the air in a perfect angle.
The aerial shell contains pellets that are arranged in a specific shape that will display when the fireworks explode in the sky. It also contains a burst charge that creates explosions in the sky.
The main fuse is ignited which lights the lift charge and results in the generation of heat and gas. This will push the aerial shell into the sky while the time delay fuse ignites. Over some time, the burst charge explodes, and the pellets burn and expand in the sky based on its arrangement.
THE CHEMISTRY OF FIREWORK POLLUTION
When the fuse is fired, the heat produced will initiate the decomposition of the reducing agents. Upon decomposition, nitrates, chlorates and perchlorates give off oxygen.
Nitrates only give up a third of their oxygen.
Chlorates get completely reduced can cause extreme explosion.
Perchlorates contain even more oxygen but are less likely to explode than chlorates due to their increase in stability.
Now, the sulfur and carbon elements in the fuel combine with the oxygen producing the hot gases, sulfur dioxide and carbon dioxide, which are air pollutants.
AIR POLLUTANTS
Sulfur dioxide and carbon dioxide are both air pollutants that are considered greenhouse gases contributing to global warming.
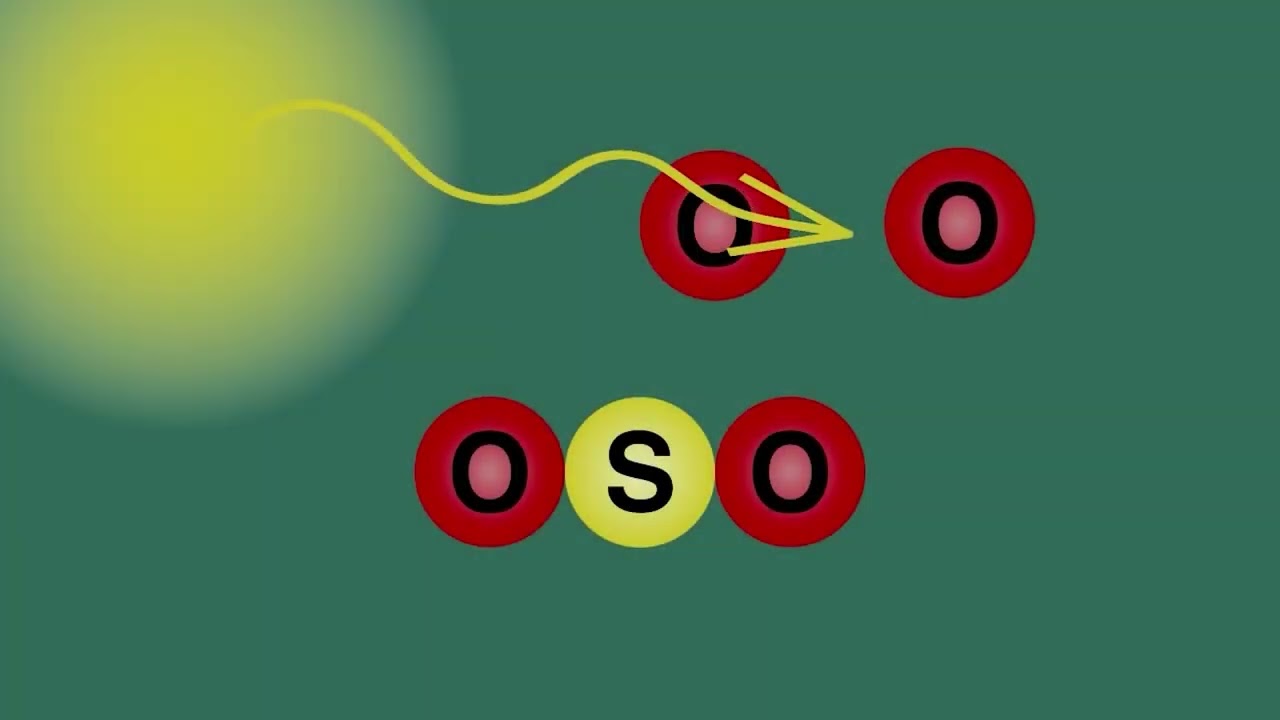
Sulfur dioxide is a bent shape molecule consists of sulfur bonded to two oxygen atoms, O=S=O.
IMPACTS:
Environmental damage-It can react with the water molecules and other chemicals present in the atmosphere to produce acid rain.
Health effects- Sulfur dioxide can also irritate the lungs and throat.
On the other hand, carbon dioxide is a linear molecule consists of a carbon atom that is doubly bonded to two oxygen atoms, O=C=O. It is a primary heat-trapping gas resulted from different anthropogenic activities.
IMPACTS:
Environmental damage- increasing CO2 reduces the earth's ability to “cool itself off”.
Health effects- headaches, dizziness, restlessness, a tingling or pins or needles feeling, difficulty breathing, sweating, tiredness, increased heart rate, elevated blood pressure, coma, asphyxia, and convulsions.
A 30-minute fireworks display leads to the projection of 3 tons of powder into the atmosphere or 1.5 tons of CO2 into the atmosphere (Painsert ng source).
Moreover, carbon monoxide may also be produced due to the incomplete burning of materials.
EFFECTS:
Environmental damage- It combines with atmospheric oxygen to increase carbon dioxide concentration in the atmosphere. his will adversely affect the environment by leading to global warming and ozone depletion.
Health effects- large amounts of CO can cause lost of consciousness and suffocation.
SOLUTION
With the emergence of progressive technology and media, the possibility of firework displays charging less than these adverse health and environmental effects might be just around the corner.
One of the most looked upon alternatives to fireworks is the illuminated drone and laser light show. More than the tingling sensation that this new form of sky art shows, it does not possess the impacts that come with burning gunpowder and metal in fireworks.
Despite the aesthetic appeal and excitement firework displays bring us, the underlying effects of its aftermath impose problems that seem to be risky for a few minutes of magical moments. With this alternative, we can have even more magical light shows, while leaving more of a healthy environment for the future generation to enjoy.
Its development dates back to the second century B.C. Bamboo stalks are considered the earliest natural firecrackers used to ward off evil spirits in ancient Liuyang, China. As a result of the hollow air pockets heating up, they explode when tossed into a fire.
In the 13th century, fireworks began to be recognized and manufactured in Europe by Italians. They were used to celebrate religious festivals, entertain and enchant the public, and illuminate the castles.
The composition inside the firework contains five (4) vital ingredients: oxidizing agent or oxidizers, fuel or reducing agent, coloring agents, and binders.
THE WORKING MECHANISM OF FIREWORKS
Fireworks are stored in a tube called mortar tube or launch tube which helps the aerial shell to sent up into the air in a perfect angle.
The aerial shell contains pellets that are arranged in a specific shape that will display when the fireworks explode in the sky. It also contains a burst charge that creates explosions in the sky.
The main fuse is ignited which lights the lift charge and results in the generation of heat and gas. This will push the aerial shell into the sky while the time delay fuse ignites. Over some time, the burst charge explodes, and the pellets burn and expand in the sky based on its arrangement.
THE CHEMISTRY OF FIREWORK POLLUTION
When the fuse is fired, the heat produced will initiate the decomposition of the reducing agents. Upon decomposition, nitrates, chlorates and perchlorates give off oxygen.
Nitrates only give up a third of their oxygen.
Chlorates get completely reduced can cause extreme explosion.
Perchlorates contain even more oxygen but are less likely to explode than chlorates due to their increase in stability.
Now, the sulfur and carbon elements in the fuel combine with the oxygen producing the hot gases, sulfur dioxide and carbon dioxide, which are air pollutants.
AIR POLLUTANTS
Sulfur dioxide and carbon dioxide are both air pollutants that are considered greenhouse gases contributing to global warming.
Sulfur dioxide is a bent shape molecule consists of sulfur bonded to two oxygen atoms, O=S=O.
IMPACTS:
Environmental damage-It can react with the water molecules and other chemicals present in the atmosphere to produce acid rain.
Health effects- Sulfur dioxide can also irritate the lungs and throat.
On the other hand, carbon dioxide is a linear molecule consists of a carbon atom that is doubly bonded to two oxygen atoms, O=C=O. It is a primary heat-trapping gas resulted from different anthropogenic activities.
IMPACTS:
Environmental damage- increasing CO2 reduces the earth's ability to “cool itself off”.
Health effects- headaches, dizziness, restlessness, a tingling or pins or needles feeling, difficulty breathing, sweating, tiredness, increased heart rate, elevated blood pressure, coma, asphyxia, and convulsions.
A 30-minute fireworks display leads to the projection of 3 tons of powder into the atmosphere or 1.5 tons of CO2 into the atmosphere (Painsert ng source).
Moreover, carbon monoxide may also be produced due to the incomplete burning of materials.
EFFECTS:
Environmental damage- It combines with atmospheric oxygen to increase carbon dioxide concentration in the atmosphere. his will adversely affect the environment by leading to global warming and ozone depletion.
Health effects- large amounts of CO can cause lost of consciousness and suffocation.
SOLUTION
With the emergence of progressive technology and media, the possibility of firework displays charging less than these adverse health and environmental effects might be just around the corner.
One of the most looked upon alternatives to fireworks is the illuminated drone and laser light show. More than the tingling sensation that this new form of sky art shows, it does not possess the impacts that come with burning gunpowder and metal in fireworks.
Despite the aesthetic appeal and excitement firework displays bring us, the underlying effects of its aftermath impose problems that seem to be risky for a few minutes of magical moments. With this alternative, we can have even more magical light shows, while leaving more of a healthy environment for the future generation to enjoy.
Комментарии
 0:07:59
0:07:59
 0:02:30
0:02:30
 0:07:32
0:07:32
 0:02:57
0:02:57
 0:10:18
0:10:18
 0:10:11
0:10:11
 0:04:37
0:04:37
 0:02:21
0:02:21
 0:02:38
0:02:38
 0:08:09
0:08:09
 0:20:57
0:20:57
 0:02:44
0:02:44
 0:09:47
0:09:47
 0:04:51
0:04:51
 0:18:32
0:18:32
 0:04:07
0:04:07
 0:29:08
0:29:08
 0:02:50
0:02:50
 0:00:11
0:00:11
 0:35:53
0:35:53
 0:03:28
0:03:28
 0:01:46
0:01:46
 0:04:07
0:04:07
 0:02:05
0:02:05