filmov
tv
Class 9: Is Matter Around Us Pure? | Science | Chapter 2 | Part 1 | Neymi
Показать описание
Neymi - Preparing students
Subject: Science (Chapter 2) Is Matter Around Us Pure?
Graphics: Yashashvi Aggarwal
Animation & Editing: Pravesh Agrawal
Recording: Nikhil Bhist (Zero Db Workstation)
Voice Over: Harsh Pandey
Special Thanks: VSFC
****************************************
This is Part 1 of Chapter 2 from class 9 Science.
We see various objects or products labelled as pure. By pure we mean, that it is unadulterated.
But for scientist “pure” means that all the particles of the substance are the same in their chemical nature. This means, a pure substance consists of a single type of particle.
Anything which is not pure and is constituted by more than one kind of particle is called a mixture. Most of the matter that we see around is a mixture. As we know mixture is made up of different atoms and molecules these are also known as “substances“. A substance can be separated into other kinds of matter by a physical process. For example: Salt/ sugar can be separated from water by various methods. As filtration, evaporation etc.
When there are no impurities in a given substance then its basic components cannot be separated from each other. Solution of NaCl and H2O is a mixture and not pure. Hence, NaCl and H2O can be separated by evaporation.
Mixtures having uniform compositions throughout are called as “Homogeneous mixtures”. For example: Salt in H2O, ink in H2O. Solutions of two or more substances are homogeneous. Mixtures having no uniform compositions and are physically distinct are called “Heterogeneous mixture”. For example: iron fillings with soil, oil and water. Heterogeneous mixture don’t mix properly.
Homogeneous mixtures of solids with liquid is called SOOL. Liquid in liquid is called emulsion. Gas with liquid is called foam. But we can also have solid SOOL known as alloys. For example: stainless steel is made up of majorly iron and some other metals to slow down or shut down rusting of iron. Similarly, we also have gaseous mixtures.
A solution is made up of a solute and a solvent. Any component which is to be mixed or dissolved into is called solute. The component in which the solute is dissolved is called the solvent. A solute is lesser in amount. A Solvent is always in larger amount. And a solution might have more than one number of solute particles.
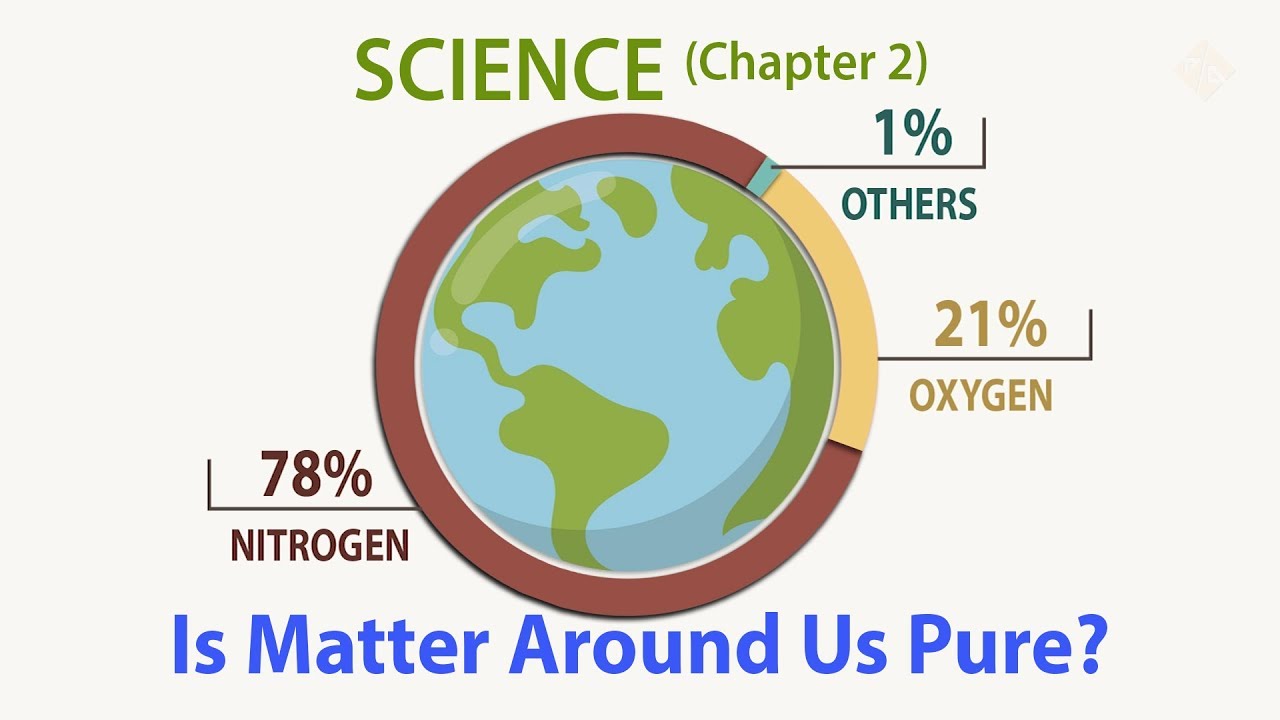
Examples: sea water has O2 dissolved in it so the life beneath can survive. Earth atmosphere consists of 21% O2 and 78% N2. And the rest is very small percentage of elements.
Properties of a true solution:
A true solution is homogeneous mixture.
Size of particles in solution is less than 1nm. (10 to the power -9 meters) in diameter, therefore cannot be seen by naked eyes.
Since the particles are so small they don’t scatter a beam of light so the path of light is not visible.
Solute cannot be separated by filtration.
The solute particles do not settle down when left undisturbed, that means the solution is stable.
Depending on the proportion of the solute to solvent, it can be termed as saturated or unsatured. We also need to understand, that the saturation point of a solution may vary at different temperatures. For example: The sugar dissolve quickly in boiling water but it takes more effort to dissolve in a cold water. When solute can be dissolved in a solvent at a given temperature, that solution is said to be a saturated solution. Where no more solute can be dissolved in a solvent at a given temperature, it is said to be an unsaturated Solution.
We can also find the concentration of a solution by a simple formula: Amount of Solute (Amount of Solution) or Amount of solute (amount of solvent).
Similarly, we can express concentration of a solution in 2 different ways:
First mass by mass percentage. In this method we use the ratio of mass of solute over mass of solution and multiply it by 100. Second Mass by volume %. In this method we use mass of solute over volume of solution multiplied by 100.
A suspension is a heterogeneous mixture in which the solute particles do not dissolve. But remain suspended throughput the bulk of the medium.
Properties of suspension are:
These particles can be seen with naked eyes.
Particles of a suspension scatter a beam of light passing through it and makes its path visible. Also known as tyndall effect.
The particles settle down when left undisturbed, that is, a suspension is unstable.
These particles can also be filtered out using filter paper.
When particles in the heterogeneous solution are spread throughout uniformly than the solution is said to be a colloid or colloidal solu
Hope you enjoy the video.
Don't forget to LIKE, SHARE and SUBSCRIBE to our channel.
*****************************************************
Email
Like Facebook Page
#Class9 #Chapter2 #Neymi
Subject: Science (Chapter 2) Is Matter Around Us Pure?
Graphics: Yashashvi Aggarwal
Animation & Editing: Pravesh Agrawal
Recording: Nikhil Bhist (Zero Db Workstation)
Voice Over: Harsh Pandey
Special Thanks: VSFC
****************************************
This is Part 1 of Chapter 2 from class 9 Science.
We see various objects or products labelled as pure. By pure we mean, that it is unadulterated.
But for scientist “pure” means that all the particles of the substance are the same in their chemical nature. This means, a pure substance consists of a single type of particle.
Anything which is not pure and is constituted by more than one kind of particle is called a mixture. Most of the matter that we see around is a mixture. As we know mixture is made up of different atoms and molecules these are also known as “substances“. A substance can be separated into other kinds of matter by a physical process. For example: Salt/ sugar can be separated from water by various methods. As filtration, evaporation etc.
When there are no impurities in a given substance then its basic components cannot be separated from each other. Solution of NaCl and H2O is a mixture and not pure. Hence, NaCl and H2O can be separated by evaporation.
Mixtures having uniform compositions throughout are called as “Homogeneous mixtures”. For example: Salt in H2O, ink in H2O. Solutions of two or more substances are homogeneous. Mixtures having no uniform compositions and are physically distinct are called “Heterogeneous mixture”. For example: iron fillings with soil, oil and water. Heterogeneous mixture don’t mix properly.
Homogeneous mixtures of solids with liquid is called SOOL. Liquid in liquid is called emulsion. Gas with liquid is called foam. But we can also have solid SOOL known as alloys. For example: stainless steel is made up of majorly iron and some other metals to slow down or shut down rusting of iron. Similarly, we also have gaseous mixtures.
A solution is made up of a solute and a solvent. Any component which is to be mixed or dissolved into is called solute. The component in which the solute is dissolved is called the solvent. A solute is lesser in amount. A Solvent is always in larger amount. And a solution might have more than one number of solute particles.
Examples: sea water has O2 dissolved in it so the life beneath can survive. Earth atmosphere consists of 21% O2 and 78% N2. And the rest is very small percentage of elements.
Properties of a true solution:
A true solution is homogeneous mixture.
Size of particles in solution is less than 1nm. (10 to the power -9 meters) in diameter, therefore cannot be seen by naked eyes.
Since the particles are so small they don’t scatter a beam of light so the path of light is not visible.
Solute cannot be separated by filtration.
The solute particles do not settle down when left undisturbed, that means the solution is stable.
Depending on the proportion of the solute to solvent, it can be termed as saturated or unsatured. We also need to understand, that the saturation point of a solution may vary at different temperatures. For example: The sugar dissolve quickly in boiling water but it takes more effort to dissolve in a cold water. When solute can be dissolved in a solvent at a given temperature, that solution is said to be a saturated solution. Where no more solute can be dissolved in a solvent at a given temperature, it is said to be an unsaturated Solution.
We can also find the concentration of a solution by a simple formula: Amount of Solute (Amount of Solution) or Amount of solute (amount of solvent).
Similarly, we can express concentration of a solution in 2 different ways:
First mass by mass percentage. In this method we use the ratio of mass of solute over mass of solution and multiply it by 100. Second Mass by volume %. In this method we use mass of solute over volume of solution multiplied by 100.
A suspension is a heterogeneous mixture in which the solute particles do not dissolve. But remain suspended throughput the bulk of the medium.
Properties of suspension are:
These particles can be seen with naked eyes.
Particles of a suspension scatter a beam of light passing through it and makes its path visible. Also known as tyndall effect.
The particles settle down when left undisturbed, that is, a suspension is unstable.
These particles can also be filtered out using filter paper.
When particles in the heterogeneous solution are spread throughout uniformly than the solution is said to be a colloid or colloidal solu
Hope you enjoy the video.
Don't forget to LIKE, SHARE and SUBSCRIBE to our channel.
*****************************************************
Like Facebook Page
#Class9 #Chapter2 #Neymi
Комментарии
 1:17:03
1:17:03
 1:16:27
1:16:27
 0:07:17
0:07:17
 0:36:02
0:36:02
 0:47:57
0:47:57
 0:14:35
0:14:35
 0:32:23
0:32:23
 0:51:54
0:51:54
 0:43:42
0:43:42
 0:24:24
0:24:24
 0:20:08
0:20:08
 0:26:41
0:26:41
 1:45:42
1:45:42
 1:21:40
1:21:40
 0:49:44
0:49:44
 1:03:26
1:03:26
 0:29:12
0:29:12
 0:57:15
0:57:15
 1:21:54
1:21:54
 0:30:45
0:30:45
 0:06:15
0:06:15
 1:50:13
1:50:13
 1:30:59
1:30:59
 1:44:49
1:44:49