filmov
tv
The Periodic Table of the Elements in Chemistry - [1-2-12]
Показать описание
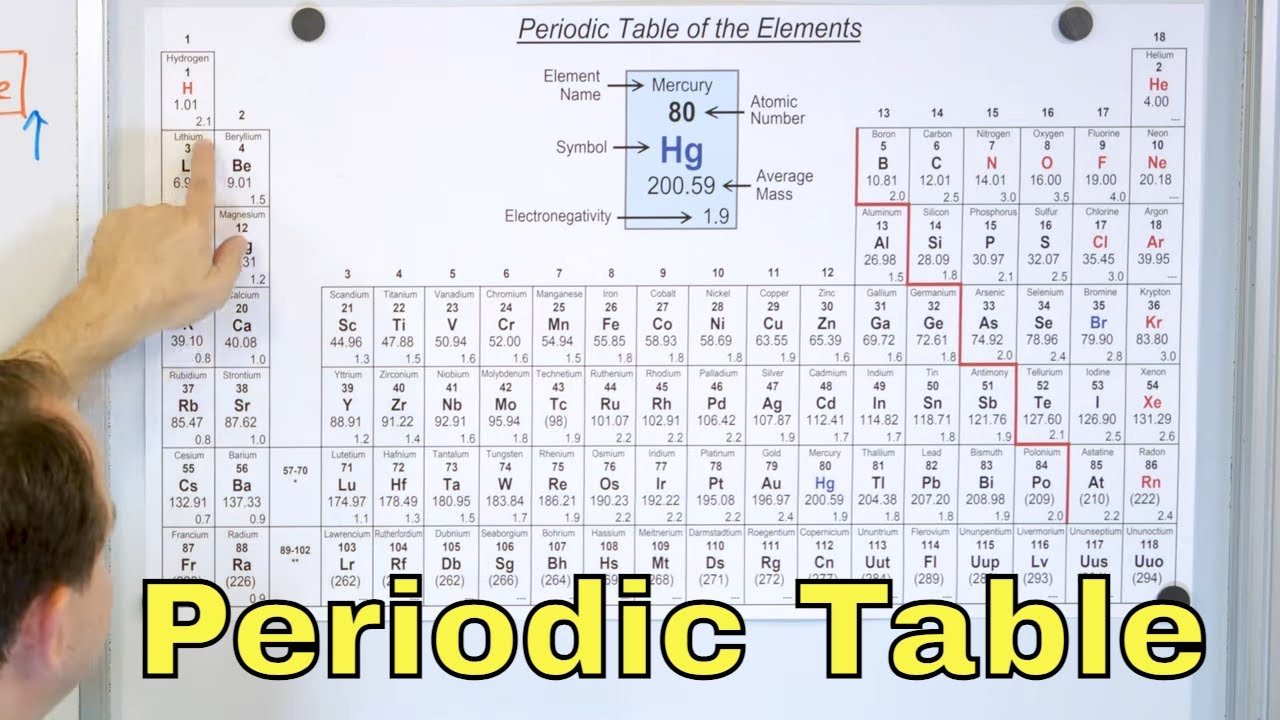
In this lesson, you will learn about the periodic table and how it is used in chemistry. We will focus on how the periodic table is organized by atomic number, and discuss how properties of elements are periodic in nature throughout the table due to the electrons in the outer shells of the atoms. We will also discuss atomic mass and valence electrons, including predicting when an atom will gain or lose an electron in a chemical reaction..
The Periodic Table Song (2018 Update!) | SCIENCE SONGS
The Periodic Table Song | SCIENCE SONGS
The Periodic Table Explained
The Periodic Table: Crash Course Chemistry #4
The genius of Mendeleev's periodic table - Lou Serico
Periodic Table Explained: Introduction
GCSE Chemistry - Modern Periodic Table #9
The Periodic Table: Atomic Radius, Ionization Energy, and Electronegativity
Explore the Fascinating World of the Periodic Table (Lec 08) NEET 2024-25
The Periodic Table Song.. ( credit goes to the rightful owner)
The Periodic Table Song Lyrics (In Order)
Perfecting the Periodic Table
The Periodic Table Song with real elements
Periodic Table Elements by Countryballs
Periodic Table
The most dangerous elements on the periodic table - Shannon Odell
How Does The Periodic Table Work | Properties of Matter | Chemistry | FuseSchool
The periodic table | Atoms, elements, and the periodic table | High school chemistry | Khan Academy
How to Read the Periodic Table
Chemistry: Introduction to the Periodic Table - Dmitri Mendeleev
What Are Periods & Groups In The Periodic Table? | Properties of Matter | Chemistry | FuseSchool
The NEW Periodic Table Song (2017) [LYRICS]
Investigating the Periodic Table with Experiments - with Peter Wothers
Solving the puzzle of the periodic table - Eric Rosado
Комментарии
 0:03:05
0:03:05
 0:02:54
0:02:54
 0:03:07
0:03:07
 0:11:22
0:11:22
 0:04:25
0:04:25
 0:14:14
0:14:14
 0:05:36
0:05:36
 0:07:53
0:07:53
 0:50:07
0:50:07
 0:00:31
0:00:31
 0:02:45
0:02:45
 0:04:31
0:04:31
 0:02:42
0:02:42
 0:02:44
0:02:44
 0:24:09
0:24:09
 0:04:39
0:04:39
 0:04:31
0:04:31
 0:08:56
0:08:56
 0:03:36
0:03:36
 0:09:06
0:09:06
 0:03:04
0:03:04
 0:02:45
0:02:45
 1:25:34
1:25:34
 0:04:19
0:04:19