filmov
tv
How To Draw The Lewis Structure of CO3 2- (Carbonate Ion) - Chemistry

Показать описание
This chemistry video tutorial explains how to draw the lewis structure of CO3 2- also known as the carbonate ion. This video discusses the resonance structure of this polyatomic ion as well as the bond angle, hybridization, and molecular geometry.
How To Draw Lewis Structures:
VSEPR Theory:
Molecular Geometry:
Lewis Dot Structures:
Lewis Structures of Ionic Compounds:
_________________________________
Octet Rule Exceptions:
Resonance Structures:
Polar and Nonpolar Molecules:
Formal Charge Calculations:
Lewis Structures - Mega Review:
________________________________
Hybridization of Atomic Orbitals:
Molecular Orbital Theory:
Dipole Dipole Forces of Attraction:
Hydrogen Bonding:
Unit Cell Chemistry:
_________________________________
Final Exams and Video Playlists:
Full-Length Videos and Worksheets:
How To Draw Lewis Structures:
VSEPR Theory:
Molecular Geometry:
Lewis Dot Structures:
Lewis Structures of Ionic Compounds:
_________________________________
Octet Rule Exceptions:
Resonance Structures:
Polar and Nonpolar Molecules:
Formal Charge Calculations:
Lewis Structures - Mega Review:
________________________________
Hybridization of Atomic Orbitals:
Molecular Orbital Theory:
Dipole Dipole Forces of Attraction:
Hydrogen Bonding:
Unit Cell Chemistry:
_________________________________
Final Exams and Video Playlists:
Full-Length Videos and Worksheets:
Комментарии
 0:11:50
0:11:50
 0:04:41
0:04:41
 0:07:26
0:07:26
 0:05:57
0:05:57
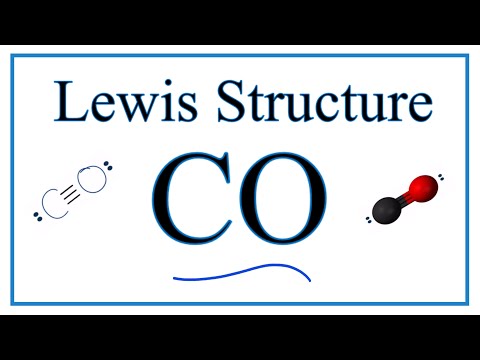 0:02:33
0:02:33
 0:11:55
0:11:55
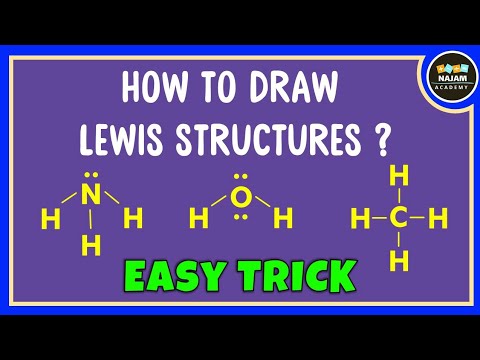 0:06:19
0:06:19
 0:06:23
0:06:23
 0:08:11
0:08:11
 0:10:29
0:10:29
 0:09:39
0:09:39
 0:01:20
0:01:20
 0:11:57
0:11:57
 0:01:16
0:01:16
 0:01:59
0:01:59
 0:02:11
0:02:11
 0:07:08
0:07:08
 0:01:22
0:01:22
 0:10:37
0:10:37
 1:07:05
1:07:05
 0:01:30
0:01:30
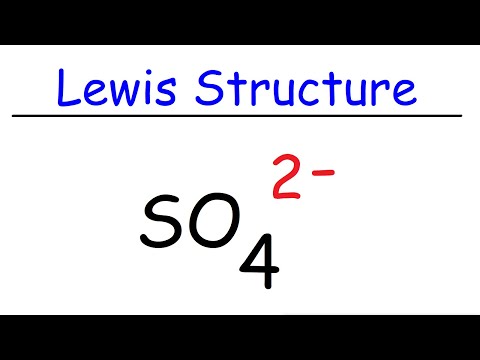 0:06:26
0:06:26
 0:01:00
0:01:00
 0:05:52
0:05:52