filmov
tv
Thermodynamics. Isothermal, Adiabatic and Diathermic terms.

Показать описание
In this tutorial we go over the terms Isothermal, Adiabatic and Diathermic systems in connection with isolated and closed systems. For more videos on thermodynamics visit Epistemeo on:
Thermodynamics. Isothermal, Adiabatic and Diathermic terms.
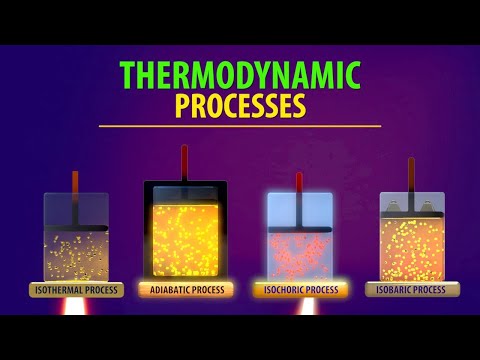
Thermodynamic Processes (Animation)
Adiabatic Process ( Classroom Demonstration)
Adiabatic Expansion: Cloud in the Bottle -- xmdemo 004
Adiabatic processes
Adiabatic & Isothermal Processes Compared
Adiabatic and Isothermal work
Isothermal and Adiabatic conceptualized
Isothermal and Adiabatic Processes
Thermodynamics 06 || Isothermal and Adiabatic Process With Best Numericals JEE MAINS/NEET
Selectivity: Isothermal vs. Adiabatic (Review)
PV diagrams part 2 (isothermal, isometric, adiabatic processes)
Isothermal Animation
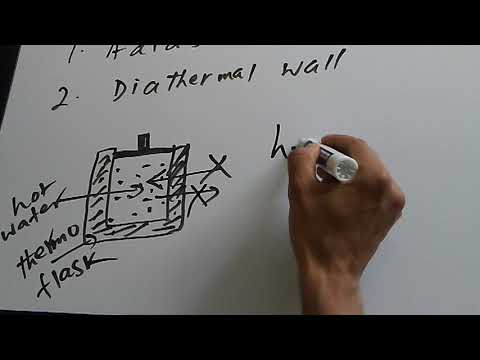
Adiabatic and Diathermal wall
Adiabatic Heating Demo
COMPARISON OF iSOTHERMAL AND ADIABATIC PROCESS-/11 chemistry/full animation
Thermodynamics : first law, isothermal and adiabatic process
Thermodynamics -1 | thermodynamic system | diathermic wall | adiabatic wall | Physics| Ombir Jindher
Adiabatic Cooling Animation
Chemical Thermodynamics: Lecture 3 - Comparison of Isothermal & Adiabatic Processes | Problem So...
Define Isothermal & Adiabatic Processes
Isothermal Expansion/Compression
Work in Isothermal,Adiabatic,Isochoric,Isobaric in Thernodynamics for IIT/Medical
First Law of Thermodynamics . Diathermal walls Adiabatic walls
Комментарии
 0:03:25
0:03:25
 0:09:19
0:09:19
 0:03:39
0:03:39
 0:00:38
0:00:38
 0:03:58
0:03:58
 0:06:15
0:06:15
 0:57:37
0:57:37
 0:19:32
0:19:32
 0:10:42
0:10:42
 1:44:44
1:44:44
 0:03:33
0:03:33
 0:13:00
0:13:00
 0:00:10
0:00:10
 0:03:36
0:03:36
 0:02:05
0:02:05
 0:03:54
0:03:54
 0:02:43
0:02:43
 0:09:36
0:09:36
 0:00:10
0:00:10
 0:22:46
0:22:46
 0:01:49
0:01:49
 0:01:36
0:01:36
 1:05:31
1:05:31
 0:04:58
0:04:58