filmov
tv
What is the Zeroth Law of Thermodynamics?
Показать описание
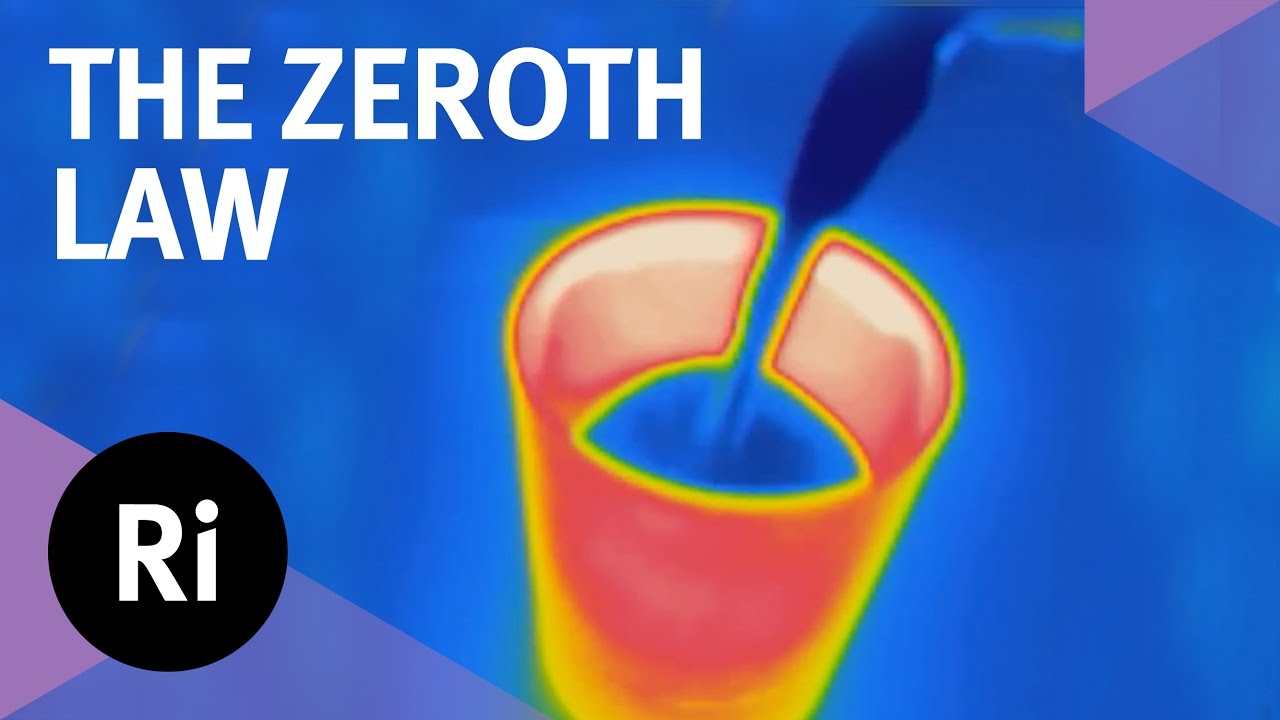
Why is there a zeroth law of thermodynamics? What use is such a simple-sounding law? And how can it be used to smash glass? Chemical engineer Valeska Ting explains in the first film from our 2016 advent calendar, all about thermodynamics.
The first, second and third laws of thermodynamics get all the glory. They’re the most well known and frequently mentioned. But underpinning them all is a final law so fundamental that, although it was established last, had to be moved to the front of the list: the zeroth law. In the first film of our 2016 advent calendar, chemical engineer Valeska Ting explores the zeroth law of thermodynamics.
The zeroth law is essentially an observation: if two systems are both in thermal equilibrium with a third, they are also in equilibrium with each other. This seemingly simple mantra is essential to our concept of temperature, as Valeska, armed with some very hot glasses, explains.
The first, second and third laws of thermodynamics get all the glory. They’re the most well known and frequently mentioned. But underpinning them all is a final law so fundamental that, although it was established last, had to be moved to the front of the list: the zeroth law. In the first film of our 2016 advent calendar, chemical engineer Valeska Ting explores the zeroth law of thermodynamics.
The zeroth law is essentially an observation: if two systems are both in thermal equilibrium with a third, they are also in equilibrium with each other. This seemingly simple mantra is essential to our concept of temperature, as Valeska, armed with some very hot glasses, explains.
The Zeroth Law of Thermodynamics: Thermal Equilibrium
ZEROTH LAW OF THERMODYNAMICS | Simple & Basic Animation
What is the Zeroth Law of Thermodynamics?
Zeroth Law of Thermodynamics
The Zeroth Law of Thermodynamics | Examples and Applications | Akash Tyagi | Embibe
Zeroth law of thermodynamics / Fourth law of thermodynamics
The First & Zeroth Laws of Thermodynamics: Crash Course Engineering #9
Zeroth law of thermodynamics | Chemical Processes | MCAT | Khan Academy
Thermodynamics Physics Class 11 | Day-1 | Zeroth law | First law of thermodynamics | Rakesh Pandey
11 chap 12 || Thermodynamics 01 || Introduction ,Thermal Equilibrium n Zeroth Law of Thermodynamics
Zeroth Law of Thermodynamics
What is Zeroth Law of Thermodynamics? | Skill-Lync
What is Zeroth Law of Thermodynamics? | Class 11 - Physics | CBSE Board | Home Revise
Zeroth Law of Thermodynamics
Zeroth Law Introduction
Zeroth Law of Thermodynamics #shorts
First Law, Second Law, Third Law, Zeroth Law of Thermodynamics
Zeroth law of thermodynamics explained #physicsdaily #thermodynamics #engineeringphysics #iitjee
Thermal Equilibrium and Zeroth Law of Thermodynamics | in HINDI
The Zeroth Law // Thermodynamics - Class 5
Zeroth Law Of Thermodynamics || Laws of thermodynamics || Zeroth law of thermodynamics in hindi
Zeroth Law | 1st Law | 2nd Law of Thermodynamics | BME | BE/Btech 1st year
Zeroth Law of thermodynamics and its applications
15.2 The Zeroth Law of Thermodynamics
Комментарии
 0:03:29
0:03:29
 0:02:40
0:02:40
 0:04:00
0:04:00
 0:08:14
0:08:14
 0:00:58
0:00:58
 0:02:17
0:02:17
 0:10:05
0:10:05
 0:06:12
0:06:12
 0:28:47
0:28:47
 0:35:33
0:35:33
 0:00:43
0:00:43
 0:03:36
0:03:36
 0:01:53
0:01:53
 0:03:45
0:03:45
 0:04:09
0:04:09
 0:00:57
0:00:57
 0:01:53
0:01:53
 0:00:53
0:00:53
 0:06:21
0:06:21
 0:05:20
0:05:20
 0:09:20
0:09:20
 0:07:47
0:07:47
 0:05:28
0:05:28
 0:03:06
0:03:06