filmov
tv
How to calculate the number of moles of each element in a chemical compound.

Показать описание
This lightboard video shows you with worked examples how to calculate the number of moles for each element within a compound. This is shown with multiple examples and amounts.
Math Antics - Finding A Percent Of A Number
Finding a Percent of a Number | Calculating Percentages
How to Calculate Faster than a Calculator - Mental Maths #1
How to find out Percentage from Calculator Easy Way
How To Calculate The Number of Protons, Neutrons, and Electrons - Chemistry
How to Calculate Faster than a Calculator - Mental Math #1
How to Calculate Your Life Path Number?
Math Help : How to Calculate an Average
How to Calculate Numbers with NEGATIVE POWERS. #inverse #numbers #maths #negative #shorts
How To Calculate Faster than a Calculator
How I learned to Calculate Extremely Fast
How-to Calculate an Even Stair Rise
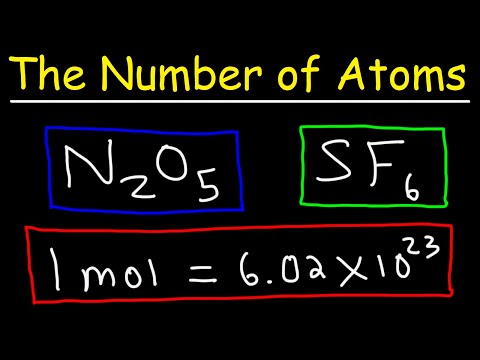
How To Calculate The Number of Atoms | Chemistry
How To Calculate The Number Needed To Treat
How to Calculate Faster than a Calculator - Mental Maths #2| Addition and Subtraction
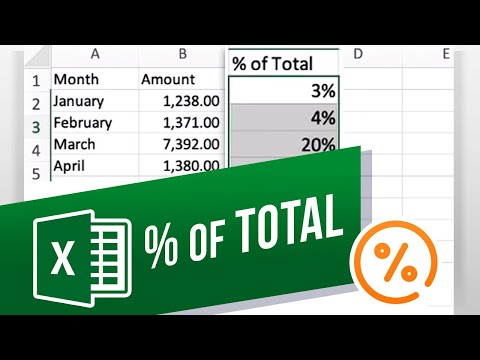
How to Calculate Percentages of Total in Excel
This is how to calculate and know your star and star number, using your date of birth by yourself.
🔮CALCULATE YOUR LIFE PURPOSE NOW • Numerology For Beginners • Beauty In Numbers • Quest for Beauty...
How To Calculate Square Roots - Numerals That Changed Math Forever
Percentage Trick | Calculate percentage in Mind | percentages made easy | zero math | in english
How to Calculate the Percentage in Excel (Formula)
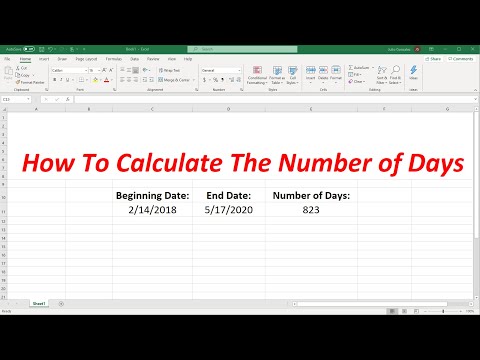
How To Calculate The Number of Days Between Two Dates In Excel
How to Calculate Square Root Without Calculator
How To Calculate Percentage In Google Sheets
Комментарии
 0:07:32
0:07:32
 0:06:27
0:06:27
 0:05:42
0:05:42
 0:02:43
0:02:43
 0:13:12
0:13:12
 0:07:20
0:07:20
 0:04:16
0:04:16
 0:01:44
0:01:44
 0:00:37
0:00:37
 0:00:30
0:00:30
 0:05:18
0:05:18
 0:00:57
0:00:57
 0:13:48
0:13:48
 0:03:48
0:03:48
 0:08:00
0:08:00
 0:01:13
0:01:13
 0:31:16
0:31:16
 0:19:17
0:19:17
 0:10:16
0:10:16
 0:06:32
0:06:32
 0:00:28
0:00:28
 0:01:18
0:01:18
 0:05:48
0:05:48
 0:00:36
0:00:36