filmov
tv
Potentiometric pH measurement

Показать описание
The pH-value of a liquid can be calculated using the potentiometric measurement principle. This video shows what it is about and how this measuring principle works.
Potentiometric pH measurement
pH Meter | working of glass electrode of pH meter
How pH SensorsWork
Potentiometric pH measurement
Chem 104 - Potentiometric pH Titration
Measurement of pH by potentiometric/electrometric method
pH meter | Principle | Study smart in minutes
Webinar recording: pH measurements made easy – Basics of potentiometric pH measurements
potentiometric pH measurement
How a pH probe works
Ask the expert: How does a pH glass electrode work?
Working Principle of pH Meter | Types of pH Meter | pH Electrode Working
Using a pH Meter
How does potentiometry work? (With real examples)
The production of pH electrodes
POTENTIOMETRIC TITRATION I INTRODUCTION I BASIC I PART-1 I HINDI
L22A Introduction to Potentiometry
[Ch 2.1] Principle of Potentiometry
QC potentiometric and pH Measurement
Potentiometric titrations (Principle, Procedure, Types, Ion-selective electrodes, applications)
Digital pH Meter = Calibration and Working Demonstration (English) | Digital pH Meter | pH Testing
pH Measurement of Cheese | Application Video
Potentiometric acid base titrations
Ion Selective Electrodes
Комментарии
 0:05:14
0:05:14
 0:09:38
0:09:38
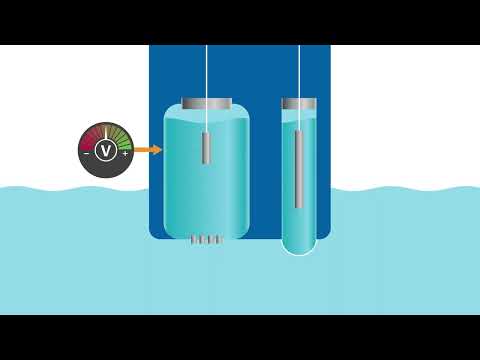 0:02:08
0:02:08
 0:02:20
0:02:20
 0:08:21
0:08:21
 0:03:16
0:03:16
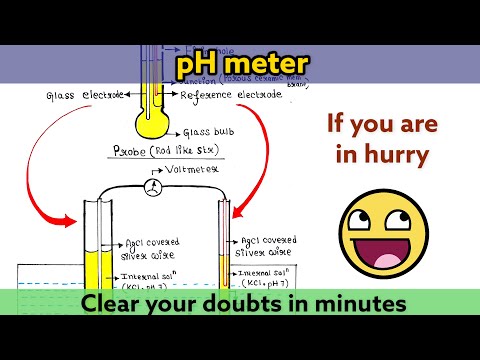 0:09:02
0:09:02
 0:47:17
0:47:17
 0:03:49
0:03:49
 0:02:31
0:02:31
 0:01:05
0:01:05
 0:02:56
0:02:56
 0:04:48
0:04:48
 0:07:00
0:07:00
 0:04:14
0:04:14
 0:08:27
0:08:27
 0:10:08
0:10:08
![[Ch 2.1] Principle](https://i.ytimg.com/vi/5MJhDzvlZCI/hqdefault.jpg) 0:05:02
0:05:02
 0:28:27
0:28:27
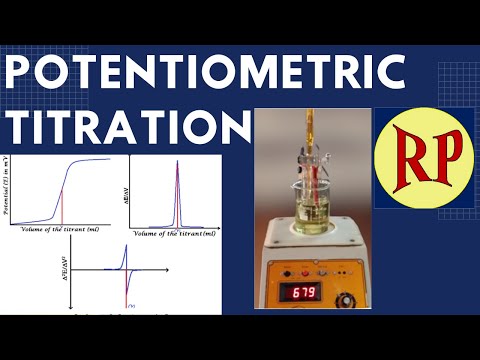 0:18:58
0:18:58
 0:05:50
0:05:50
 0:04:24
0:04:24
 0:02:30
0:02:30
 0:02:48
0:02:48