filmov
tv
ALEKS - Using the Ideal Equation of State (2 of 2 - Harder Version)

Показать описание
ALEKS: Using the ideal equation of state
ALEKS - Using the Ideal Equation of State (1 of 2 - Easier Version)
ALEKS - Using the Ideal Equation of State (2 of 2 - Harder Version)
ALEKS - Using the Ideal Equation of State (3 of 3 - harder version, shorter video)
ALEKS - Ideal Equation of State
Aleks Using the ideal equation of state
ALEKS: Using thermodynamic state to order the ideality of a gas
ALEKS: Using the combined gas law
ALEKS: Approximating the equation of a line of best fit and making predictions
ALEKS - Calculating ideal solution composition after a distillation
ALEKS: Using the van der Waals equation of state
ALEKS: Identifying the origin of nonideality in a gas
ALEKS: Interconverting molar mass and density
How to: ALEKS PPL
ALEKS: Using Charles’s Law
ALEKS - Using Kf to calculate the equilibrium molarity of a complex
ALEKS: Using the Faraday constant
Aleks Interconverting molar mass and density of ideal gas
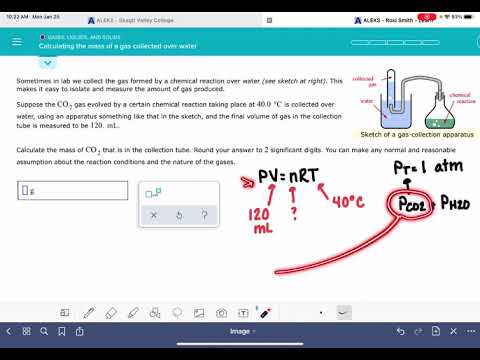
ALEKS: Calculating the mass of a gas collected over water
Aleks Understanding Boyle's Law
ALEKS: Using a phase diagram to predict a phase at a given temperature and pressure
ALEKS: Using solubility to calculate solute mass or solution volume
Aleks Using Charles's Law
ALEKS: Using Le Chatelier’s Principle to predict the result of changing concentration
Комментарии
 0:03:27
0:03:27
 0:04:20
0:04:20
 0:08:20
0:08:20
 0:06:40
0:06:40
 0:08:53
0:08:53
 0:04:35
0:04:35
 0:03:00
0:03:00
 0:06:35
0:06:35
 0:07:05
0:07:05
 0:20:38
0:20:38
 0:09:07
0:09:07
 0:04:42
0:04:42
 0:02:19
0:02:19
 0:01:16
0:01:16
 0:04:01
0:04:01
 0:12:51
0:12:51
 0:06:57
0:06:57
 0:04:52
0:04:52
 0:07:13
0:07:13
 0:04:23
0:04:23
 0:02:59
0:02:59
 0:07:03
0:07:03
 0:05:30
0:05:30
 0:03:14
0:03:14