filmov
tv
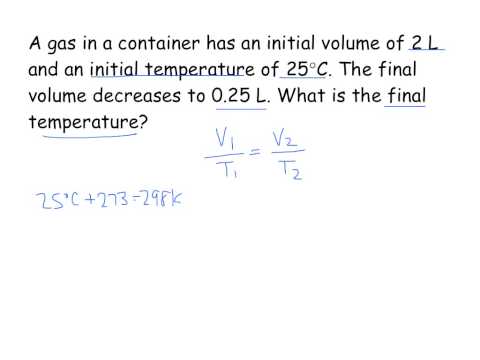
Charles's Law - Solving for Final Temperature

Показать описание
A worked Charles's Law problem that shows step by step how to solve for final temperature.
Charles' Law
Charles's Law - Solving for Final Temperature
Chemistry: Charles's Law (Gas Laws) with 2 example problems
How to Solve Charles' Law (Gas Law) Tagalog-Explained
Gas Laws-Boyle's-Charles's-Gay Lussac's
CHARLES' LAW | Animation
Charles's Law - Practice - 1
Chemistry: Boyle's Law (Gas Laws) with 2 example problems
Charles's Law: Solving for Final Volume
Boyle's Law Practice Problems
How to Use Each Gas Law | Study Chemistry With Us
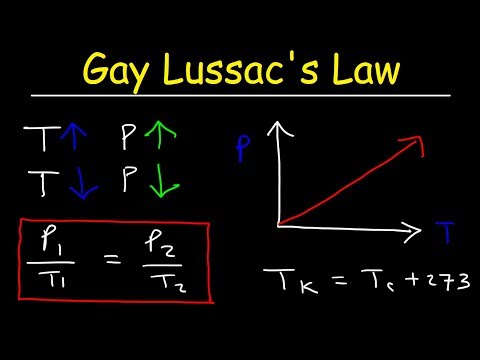
Gay Lussac's Law Practice Problems
ALEKS: Using Charles’s Law
Gas Laws: Temperature Vs. Volume; Charles law demo
Charles's Law
Chemistry 1 - gases - Charles' Law Graph
Numericals of Charles Law #shorts #youtubeshorts
Demonstration of Charles's Law
Charles's law Experiment #shorts
Grade 10 SCIENCE | Quarter 4 Module 1 | Boyle's Law and Charles's Law
Charles's law || How da? || Infinitetengineers
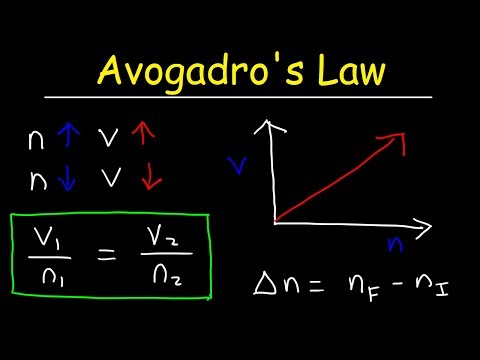
Avogadro's law Practice Problems
Charles law- why does a wish paper lantern goes up? #shorts
Chemistry: Gay-Lussac's Law (Gas Laws) with 2 example problems
Комментарии
 0:11:51
0:11:51
 0:02:19
0:02:19
 0:05:54
0:05:54
 0:10:27
0:10:27
 0:02:34
0:02:34
 0:03:21
0:03:21
 0:05:14
0:05:14
 0:05:26
0:05:26
 0:05:58
0:05:58
 0:12:25
0:12:25
 0:26:34
0:26:34
 0:13:27
0:13:27
 0:04:01
0:04:01
 0:00:25
0:00:25
 0:04:30
0:04:30
 0:02:09
0:02:09
 0:00:17
0:00:17
 0:00:10
0:00:10
 0:00:44
0:00:44
 0:33:41
0:33:41
 0:00:37
0:00:37
 0:11:04
0:11:04
 0:00:34
0:00:34
 0:05:43
0:05:43