filmov
tv
why intrinsic carrier concentration of germanium is larger than silicon ?
Показать описание
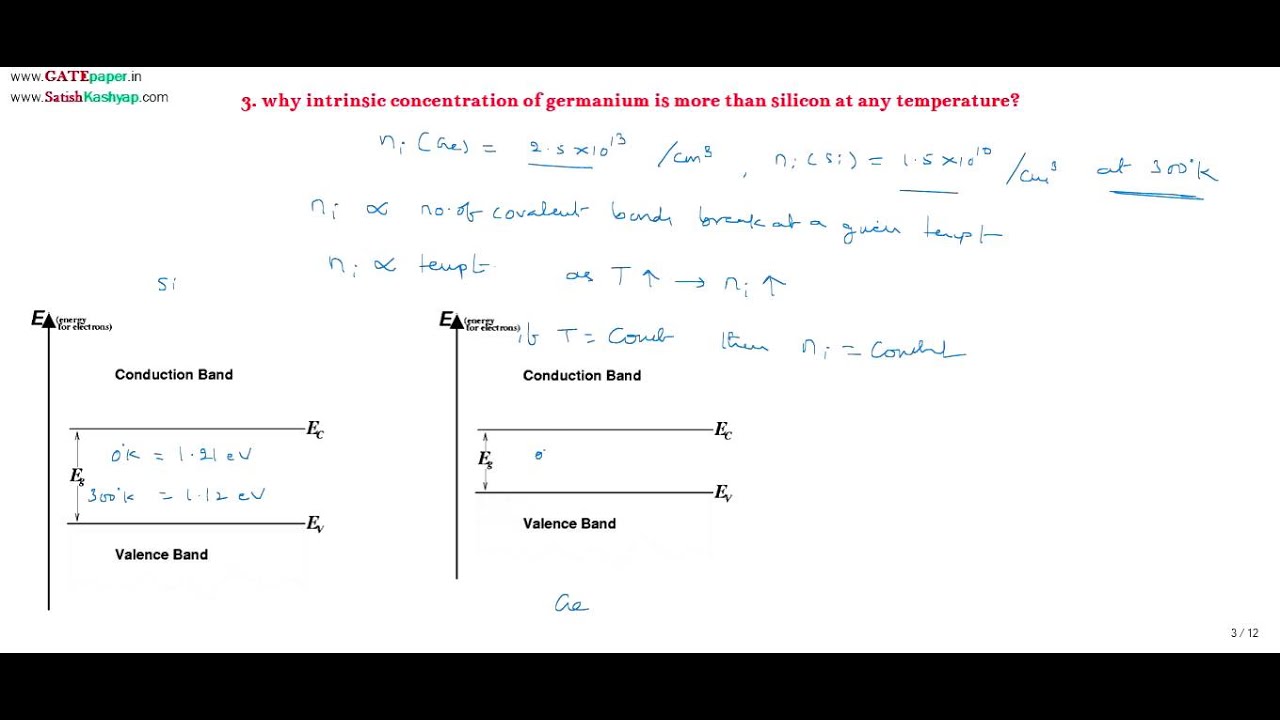
Intrinsic concentration (ni) is referred as the number of covalent bonds break at a given temperature per unit volume. It is a function of temperature, so as temperature increases, ni increases.
We know, energy gap is defined as the difference between lowest energy level of conduction band and highest energy level of valence band, and is the minimum energy required to break a covalent bond so that an electron from valence band can reach conduction band. When a covalent bond breaks, an electron in conduction band (free electron) and a vacancy in valence band (hole) is created.
Germanium atom has less energy gap than Silicon atom, so at a given temperature ie. For the given thermal energy, more covalent bonds will break in germanium than in silicon, so ni is more in Ge than Si.
We know, energy gap is defined as the difference between lowest energy level of conduction band and highest energy level of valence band, and is the minimum energy required to break a covalent bond so that an electron from valence band can reach conduction band. When a covalent bond breaks, an electron in conduction band (free electron) and a vacancy in valence band (hole) is created.
Germanium atom has less energy gap than Silicon atom, so at a given temperature ie. For the given thermal energy, more covalent bonds will break in germanium than in silicon, so ni is more in Ge than Si.
Electronic Devices Intrinsic carrier concentration
Electronic Devices: Intrinsic carrier concentration
The intrinsic carrier concentration of germanium (GE) is expressed as ni -1.66 x 10 ST - Eg m-3 x…
why intrinsic carrier concentration of germanium is larger than silicon ?
3.4 Intrinsic carrier concentration | Dr. Ramu Mannam
Doping and intrinsic carrier concentration
GATE 1987 ECE intrinsic carrier concentration
Intrinsic carrier Concentration - PART 1 - No of electrons in the CB
Concentration of charge carrier in intrinsic semiconductor
Intrinsic carrier concentration
intrensic semi conductor density of electrons DERIVATION || engineering physics ||
Carrier Concentration in intrinsic semiconductor
2.2 Intrinsic carrier density
intrinsic semi conducter carrier concentration and fermi level Engineering Physics etution
Intrinsic Semiconductor (semiconductors) PHYSICS
No of holes in the VB and Carrier Concentration of an Intrinsic Semiconductor - PART 2
If a semiconductor has an intrinsic carrier concentration of `1.41 xx 10^(16)//m^(3)` when
Charge Carrier Concentration of Doped Semiconductors
Solid State Electronics | Temperature Dependence of Carrier Concentration (Extrinsic)
Intrinsic Carrier Concentration
Solid State Electronics | Temperature Dependence of Carrier Concentration (Intrinsic)
SP & Diodes - Example: Intrinsic Carrier Concentration
Semiconductors L7: Carrier Concentration | Mass Action Law | Physics Endgame | Vikrant Kirar
Intrinsic Concentration in Semiconductor Material | Electronics Devices and Circuits - EDC
Комментарии
 0:08:59
0:08:59
 0:08:59
0:08:59
 0:12:05
0:12:05
 0:05:21
0:05:21
 0:47:04
0:47:04
 0:33:43
0:33:43
 0:02:34
0:02:34
 0:31:07
0:31:07
 0:14:54
0:14:54
 0:38:11
0:38:11
 0:18:01
0:18:01
 0:13:24
0:13:24
 0:12:08
0:12:08
 0:07:55
0:07:55
 0:16:11
0:16:11
 0:25:02
0:25:02
 0:01:44
0:01:44
 0:12:28
0:12:28
 0:05:18
0:05:18
 0:53:17
0:53:17
 0:05:58
0:05:58
 0:07:54
0:07:54
 0:08:19
0:08:19
 0:13:08
0:13:08