filmov
tv
14.2 Hybridization (HL)

Показать описание
This video covers sp, sp2 and sp3 hybridization.
14.2 Hybridization (HL)
S2.2.16 Hybridisation (HL)
S2.2.15 Explain hybridization as mixing of orbitals making new orbitals [HL IB Chemistry]
IB Chemistry Topic 14.2 (HL): Hybridisation, Delocalisation & Ozone Depletion
Hybridization - A Simple and Helpful Explanation | IB Chemistry HL 14.2
Valence Bond Theory, Hybrid Orbitals, and Molecular Orbital Theory
IB Chemistry Topic 14.2 Hybridisation sp sp2 sp3
S2.2.16 Describe σ and π bonds IB Chemistry [HL IB Chemistry]
14.1.1 Predict the shape and bond angles for species with 5 or 6 charge centers IB Chemistry HL
String Theory Explained in a Minute
Chem162 Hybridization and Examples (10.5 Part 4)
What hybridization is expected on the central atom of each of the following molecules? (i) BeH_2 ...
What is Hybridisation ANYWAY?! (IB Chemistry S2.2)
Lay Hand Prayer,Bishop Amardeep Ministry #bishopamardeepministry
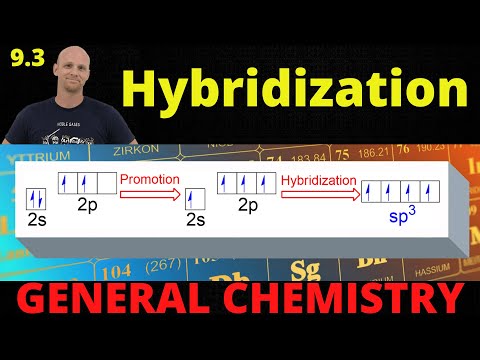
9.3 Hybridization | General Chemistry
Tricks tofindVBT,SFL,WFL,CFSE,Hybridization,unpaired electron, Magnetic moment,C.N=4#kset#chemistry
Covalent Bonding [IB Chemistry SL/HL]
How Mendel's pea plants helped us understand genetics - Hortensia Jiménez Díaz
Electron Configuration - Basic introduction
Identifying the acid and base in an acid-base reaction #acidsandbases #organicchemistry
Indicating Hybridization of Carbon Atoms 001
Hybridization(HD)
Chemistry 4.6 Orbital Hybridization
Worked examples Lewis structures+polarity+hybridization
Комментарии
 0:06:50
0:06:50
 0:06:15
0:06:15
 0:05:28
0:05:28
 0:06:01
0:06:01
 0:18:33
0:18:33
 0:07:54
0:07:54
 0:08:08
0:08:08
 0:02:09
0:02:09
 0:05:24
0:05:24
 0:00:58
0:00:58
 0:10:01
0:10:01
 0:04:10
0:04:10
 0:17:14
0:17:14
 0:00:27
0:00:27
 0:16:52
0:16:52
 0:20:31
0:20:31
 0:14:30
0:14:30
 0:03:07
0:03:07
 0:10:19
0:10:19
 0:00:34
0:00:34
 0:02:48
0:02:48
 0:06:59
0:06:59
 0:09:08
0:09:08
 0:22:34
0:22:34