filmov
tv
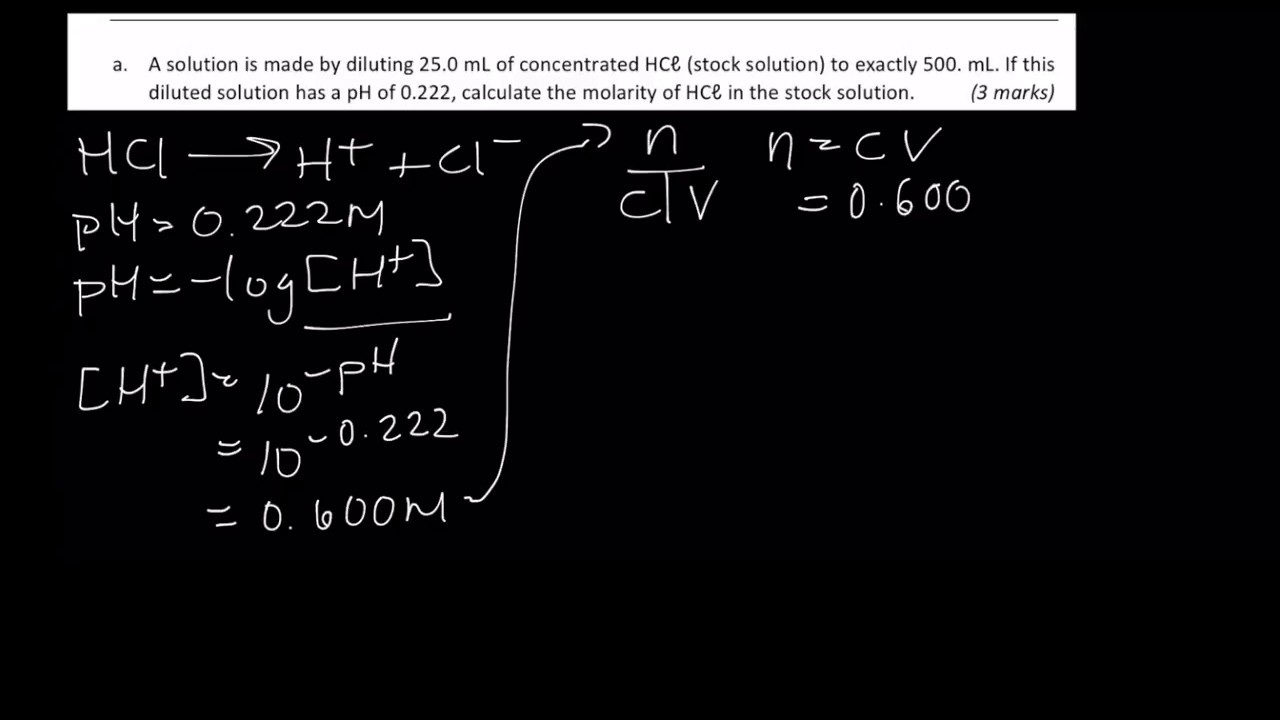
Calculating MOLARITY from pH!
Показать описание
Finding MOLARITY when given pH and pOH | Chemistry with Cat
Molarity of a strong base from pH
Molarity of strong acid from pH
Calculating MOLARITY from pH!
pH, pOH, H3O+, OH-, Kw, Ka, Kb, pKa, and pKb Basic Calculations -Acids and Bases Chemistry Problems
Finding pH and pOH using MOLARITY of a solution | Chemistry with Cat
Molarity Practice Problems
Ka from pH and a concentration
How to calculate concentration from pH and pOH
How to find pH from molarity and Ka
Molarity Made Easy: How to Calculate Molarity and Make Solutions
Calculate ph from molarity
Calculate the pH of Acids and Bases Given the Concentration of a Solution
How to find pH, pOH, H3O+, and OH- STEP BY STEP
pH of Weak Acids and Bases - Percent Ionization - Ka & Kb
How to Calculate Hydrogen Ion Concentration from pH
Molarity, Molality, Volume & Mass Percent, Mole Fraction & Density - Solution Concentration ...
How to find the Ka of an acid when given pH
Given pH & pOH, Solve for [H+] & [OH-] Practice Problems
How To Calculate The pH of a Solution Without a Calculator - Acids and Bases
Molarity and pH Calcs Less than 10 mins
Find the [OH-] and pH given the molarity of a solution
pH and pOH: Crash Course Chemistry #30
How to calculate pH Chemistry of water 💦 101 #molarity #phscale #acidity
Комментарии
 0:04:14
0:04:14
 0:02:09
0:02:09
 0:02:12
0:02:12
 0:02:45
0:02:45
 0:13:50
0:13:50
 0:07:15
0:07:15
 0:21:27
0:21:27
 0:04:23
0:04:23
 0:03:14
0:03:14
 0:03:45
0:03:45
 0:08:46
0:08:46
 0:06:48
0:06:48
 0:28:17
0:28:17
 0:04:05
0:04:05
 0:29:31
0:29:31
 0:01:57
0:01:57
 0:31:25
0:31:25
 0:04:00
0:04:00
 0:08:38
0:08:38
 0:21:09
0:21:09
 0:09:39
0:09:39
![Find the [OH-]](https://i.ytimg.com/vi/X4jUZkhGWUU/hqdefault.jpg) 0:02:23
0:02:23
 0:11:23
0:11:23
 0:00:52
0:00:52