filmov
tv
Development and Delivery of Pharmaceutical Products (CMC) - MaRS Best Practices

Показать описание
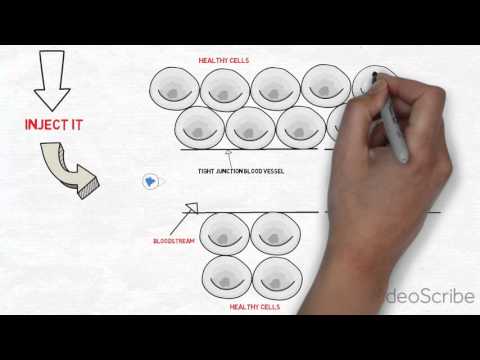
Moving from drug discovery to drug development requires a particular skillset usually not yet honed by start-ups. This phase of the development process is highly regulated and critical. Let Dr. Colin Minchom take you through the aspects of the Chemistry, Manufacturing and Control (CMC) portion of the drug development process.
Watch this video to learn about the pharmaceutical product development which includes objectives of formulation, cost-effective strategies to reach key milestones and more.
MaRS -- Building Canada's next generation of global technology companies.
Watch this video to learn about the pharmaceutical product development which includes objectives of formulation, cost-effective strategies to reach key milestones and more.
MaRS -- Building Canada's next generation of global technology companies.
Комментарии