filmov
tv
Quantum Chemistry 9.5 - Antisymmetry Principle
Показать описание
Short lecture on the antisymmetry principle for electrons.
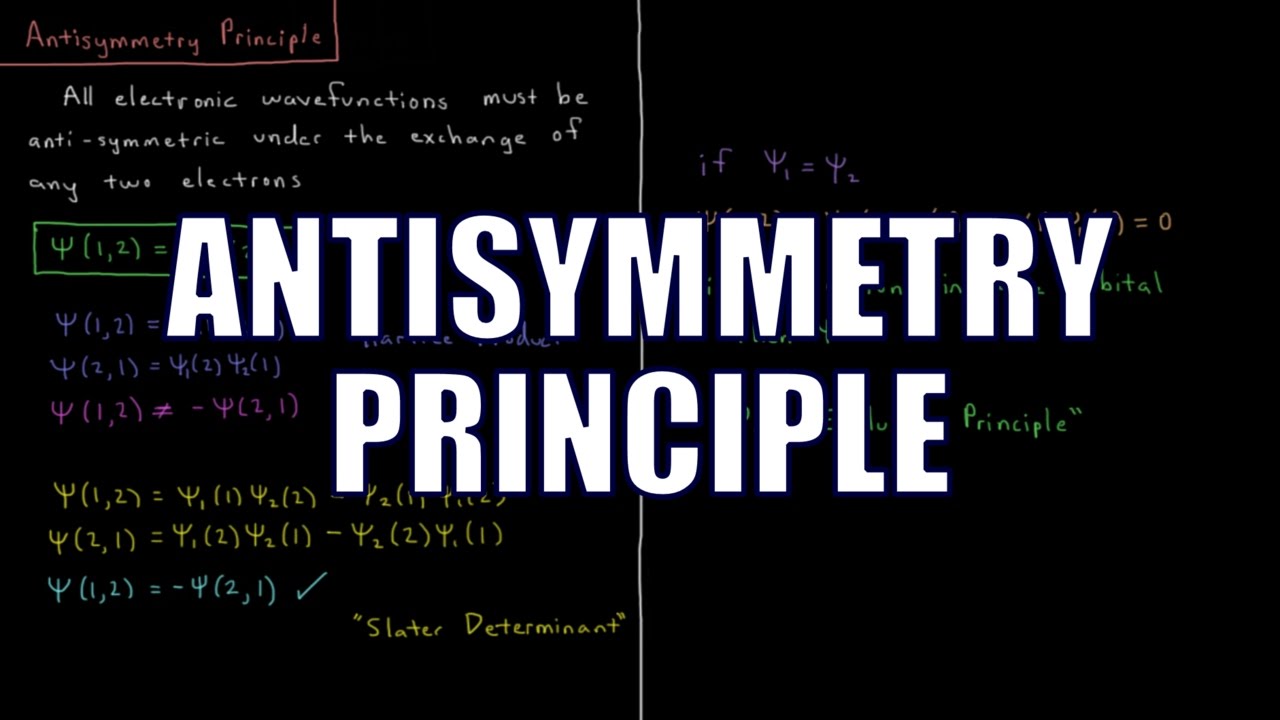
The wavefunction of any atom or molecule must switch sign when two electrons are exchanged. This anti-symmetry principle results in a total wavefunction which is a Slater determinant. This is the physical origin of the Pauli exclusion principle, which makes the wavefunction go to zero when two electrons are placed in the same spin orbital.
--- About TMP Chem ---
All TMP Chem content is free for everyone, everywhere, and created independently by Trent Parker.
--- Video Links ---
--- Social Links ---
--- Equipment ---
Microphone: Blue Yeti USB Microphone
Drawing Tablet: Wacom Intuos Pen and Touch Small
Drawing Program: Autodesk Sketchbook Express
Screen Capture: Corel Visual Studio Pro X8
The wavefunction of any atom or molecule must switch sign when two electrons are exchanged. This anti-symmetry principle results in a total wavefunction which is a Slater determinant. This is the physical origin of the Pauli exclusion principle, which makes the wavefunction go to zero when two electrons are placed in the same spin orbital.
--- About TMP Chem ---
All TMP Chem content is free for everyone, everywhere, and created independently by Trent Parker.
--- Video Links ---
--- Social Links ---
--- Equipment ---
Microphone: Blue Yeti USB Microphone
Drawing Tablet: Wacom Intuos Pen and Touch Small
Drawing Program: Autodesk Sketchbook Express
Screen Capture: Corel Visual Studio Pro X8
F2020 552 Lecture 5 (Chapter 14, Part 5) Sep 11, 2020
Symmetric and Anti-symmetric Wave functions
Antisymmetric wave function|Space and spin part of wavefunction|NET Previous year questions quantum
Quantum Chemistry 9.0 - Many-Electron Atoms Review (Old Version)
Constructing Symmetric & Antisymmetric Functions by Nidhi Sharma from Quantum Mechanics
Pauli Exclusion Principle
Quantum Chemistry 9.10 - Hartree-Fock Spin (Old Version)
Quantum Chemistry 9.12 - Atomic Electron Configurations (Old Version)
Quantum Chemistry 12.0 - Symmetry and Group Theory Review (Old Version)
Quantum Chemistry 0.0 - Course Review (Old Version)
Quantum Chemistry 9.18 - Hund's Rules (Old Version)
ANTISYMMETRIC NATURE OF WAVEFUNCTION FOR FERMIONS
Quantum Chemistry 9.7 - Hartree-Fock Atomic Energy (Old Version)
Lecture 9: Multi-Electron Atoms
Quantum Chemistry 1.0 - Early Quantum Review (Old Version)
Quantum Chemistry
22 1 Good quantum numbers, terms, levels, and states
6: Postulates of Quantum Mechanics (3, 4 and 5); Expectation Value
Quantum Chemistry 9.8 - Hartree-Fock Operators (Old Version)
Quantum Chemistry: 5 Types of Questions Which Everyone can Solve | CSIR NET | GATE | IIT JAM
Analysis of the Extended Coupled-Cluster Method in Quantum Chemistry
Quantum Chemistry - Lecture 10.2
Quantum Chemistry 9.3 - Hartree-Fock Helium Atom (Old Version)
QM|HAP|L5|Hydrogen Atom Orbitals
Комментарии
 0:50:58
0:50:58
 0:13:12
0:13:12
 0:23:37
0:23:37
 0:09:00
0:09:00
 0:03:43
0:03:43
 0:08:23
0:08:23
 0:12:39
0:12:39
 0:09:03
0:09:03
 0:06:26
0:06:26
 0:12:40
0:12:40
 0:05:57
0:05:57
 0:15:55
0:15:55
 0:11:57
0:11:57
 0:42:40
0:42:40
 0:05:37
0:05:37
 0:55:15
0:55:15
 0:49:57
0:49:57
 0:19:28
0:19:28
 0:11:04
0:11:04
 0:28:08
0:28:08
 0:44:52
0:44:52
 0:44:52
0:44:52
 0:11:21
0:11:21
 0:49:10
0:49:10