filmov
tv
H2O2 + MnO2 (Hydrogen peroxide + Manganese dioxide)
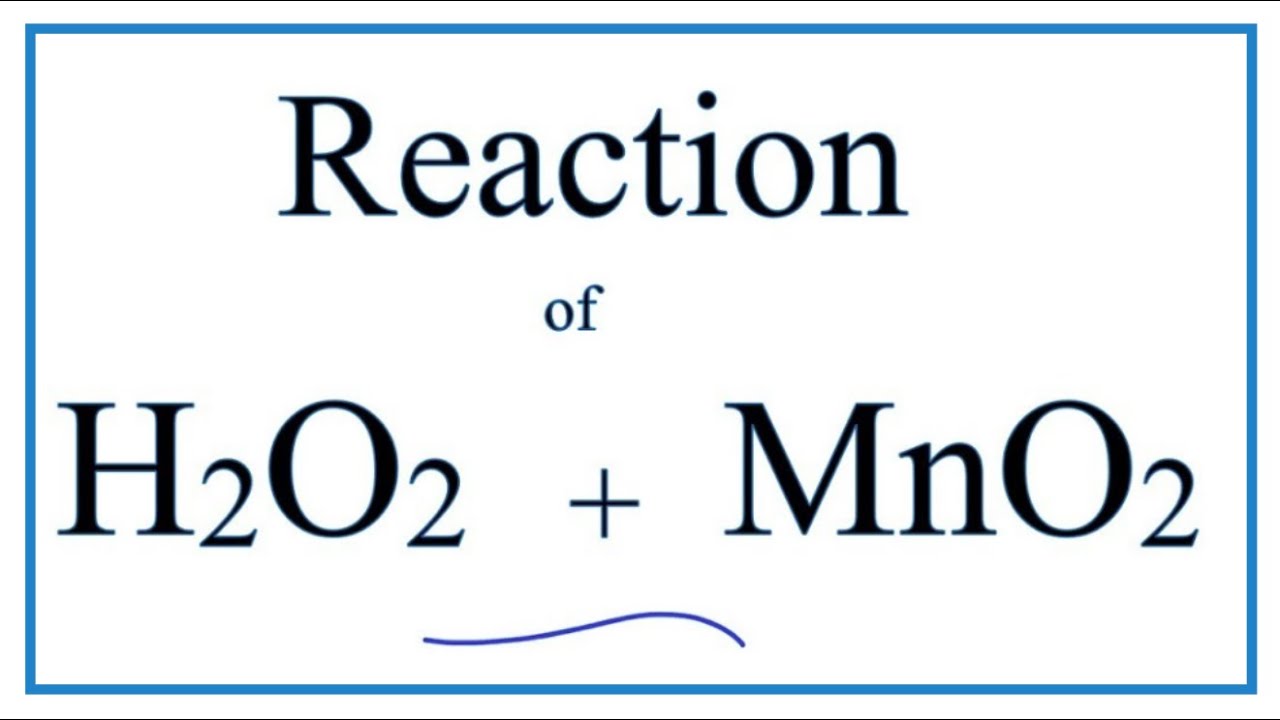
Показать описание
H2O2 (hydrogen perioxide) will decompose into H2O and O2 on its own, albeit slowly. One reason H2O2 comes in dark bottles is to slow this process, which is accelerated by light. To catalyze, or speed up the reaction, we can add MnO2 (Manganese dioxide) to the H2O2. The MnO2 isn’t changed as a result of the reaction and is not considered a reactant. Instead, we write MnO2 above the “yields” arrow to show that it served as a catalyst in the reaction.
There is also an enzyme in your blood called catalase. That is why you see bubbles when you put H2O2 on a wound. The bubbles are O2 gas. Note that enzymes are considered biological catalysts.
There is also an enzyme in your blood called catalase. That is why you see bubbles when you put H2O2 on a wound. The bubbles are O2 gas. Note that enzymes are considered biological catalysts.
H2O2 + MnO2 (Hydrogen peroxide + Manganese dioxide)
Decomposition of hydrogen peroxide with Manganese dioxide
Fantastic!!!!How to produce Oxygen(O2) with Hydrogen peroxide (H2O2) and MnO2
KMnO4 + H2O2 #hydrogenperoxide #H2O2 #bluebox #chemistry #kmno4 #potassiumpermanganate #nilered #o2
Hydrogen peroxide + Manganese dioxide 🔥🧪 #shorts
H2O2 and MnO2
Hydrogen Peroxide Decomposition (with and without MnO2 catalyst)
Reacting hydrogen peroxide with manganese dioxide
Decomposition of H2O2 with MnO2
Decomposition of H2O2 (Details in description) #experiment #reactionshorts #science #fun #magic
MnO2 + H2O2 Reaction (Manganese dioxide + Hydrogen peroxide)
Decomposition of H2O2 in presence of MnO2.
MnO2 + 10ml 6% H2O2 + 10 ml water SET-UP
Genie in a Bottle- Concentrations H2O2 and MnO2
Genie in the Bottle! (MnO2 Catalyzes the Decomposition of H2O2) in RamZland!⚗️
Sticken With Science - Decomposition of Hydrogen Peroxide With MnO2
Hydrogen peroxide and manganese dioxide reaction
iodine and Hydrogen peroxide reaction experiment #shorts #scienceexperiment
H2O2 and MnO2
How to make an Oxygen Generator (MnO2/H2O2 Method)
MnO2 + 10ml 6% H2O2 + 10ml water EXPERIMENT
Reaction Of Hydrogen Peroxide And Manganese Dioxide
Hydrogen Peroxide and MnO2
Hydrogen peroxide and manganese dioxide experiment 3 C0094
Комментарии
 0:01:15
0:01:15
 0:00:26
0:00:26
 0:01:42
0:01:42
 0:00:35
0:00:35
 0:00:11
0:00:11
 0:00:40
0:00:40
 0:04:53
0:04:53
 0:00:56
0:00:56
 0:04:06
0:04:06
 0:00:17
0:00:17
 0:00:26
0:00:26
 0:00:13
0:00:13
 0:01:19
0:01:19
 0:02:45
0:02:45
 0:01:07
0:01:07
 0:01:51
0:01:51
 0:00:26
0:00:26
 0:00:23
0:00:23
 0:00:25
0:00:25
 0:03:11
0:03:11
 0:06:37
0:06:37
 0:00:21
0:00:21
 0:12:47
0:12:47
 0:10:32
0:10:32