filmov
tv
Naming Amines for A-level Chemistry using IUPAC rules

Показать описание
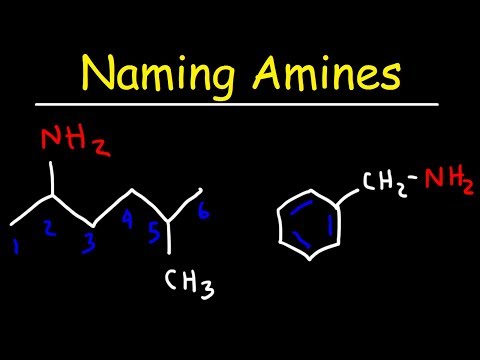
The alternative naming system for structure 1 for example would be butylamine as opposed to butan-1-amine like I have described in the video. According to OCR (via a tweet to me) they would accept wither in the A-level exams BUT the IUPAC version (described in this video) is now preferred.
Also ... I am fully aware that some of my molymod strucutres were missing C-H groups (haha), but thanks to Alexa responding to certain words in this tutorial, it already took way longer than expected to record/edit it so I decided to leave them.
Topics are organised by OCR module in the correct sequence of the specification.
Please like this video if you use this resource and consider subscribing to stay updated and show your support! Thanks so much everyone.
Also ... I am fully aware that some of my molymod strucutres were missing C-H groups (haha), but thanks to Alexa responding to certain words in this tutorial, it already took way longer than expected to record/edit it so I decided to leave them.
Topics are organised by OCR module in the correct sequence of the specification.
Please like this video if you use this resource and consider subscribing to stay updated and show your support! Thanks so much everyone.
Naming Amines for A-level Chemistry using IUPAC rules
Naming Amines - IUPAC Nomenclature & Common Names
Amines and Amides
Primary, Secondary and Tertiary Amines using IUPAC Systematic nomenclature - A-Level Chemistry
Names of Amines #science #chemistry #amines
Amine naming introduction | Amines | Organic chemistry | Khan Academy
Naming of Amines and Nitriles
AQA A-Level Chemistry - Amines
Quick Revision - Amines
AQA 3.11 Amines REVISION
Naming Amines using IUPAC Nomenclature for Organic Compounds by Leah4sci
Amines - naming and reactions
Amines: Crash Course Organic Chemistry #46
Amines | Amines & Amides | A level Chemistry 9701
Amines & Phenylamine | Organic chemistry | 9701 A Level Chemistry
22.1 Naming Amines | Organic Chemistry
Amines and Amides Organic chemistry A level AQA Chemistry
Amine naming introduction | Amines | Organic chemistry | Khan Academy
Introduction to amines
Naming Primary Amines
Amines - Basicity (A-level Chemistry)
Amine Synthesis | Reactants | MCQ
Amines - Preparation (A-Level IB Chemistry)
10.1 Naming amines (SL)
Комментарии
 0:09:37
0:09:37
 0:18:06
0:18:06
 0:04:09
0:04:09
 0:04:14
0:04:14
 0:05:02
0:05:02
 0:06:51
0:06:51
 0:03:52
0:03:52
 0:30:52
0:30:52
 0:08:08
0:08:08
 0:29:49
0:29:49
 0:07:09
0:07:09
 0:14:52
0:14:52
 0:12:11
0:12:11
 0:52:02
0:52:02
 0:15:54
0:15:54
 0:06:54
0:06:54
 0:04:57
0:04:57
 0:05:43
0:05:43
 0:08:04
0:08:04
 0:06:51
0:06:51
 0:10:43
0:10:43
 0:01:00
0:01:00
 0:12:32
0:12:32
 0:03:10
0:03:10