filmov
tv
L-9: Concept of back bonding in boron trifluoride

Показать описание
#bigbangchemistry #BF3 # PpiPpibackbonding #Boronhalides
#NEET
#IITJEE
#JEEMAIN
#ConceptofbackbondinginBorontrluoride
#Hybridization
#Chemical Bonding for class 11
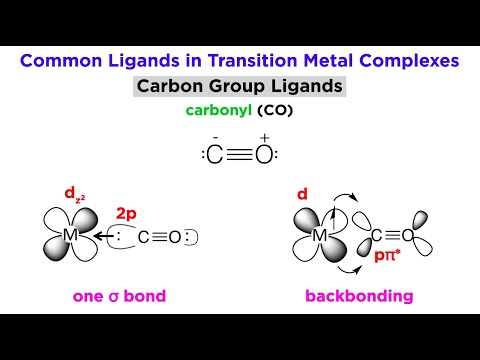
concept of back bonding in boron trifluoride: Boron has an empty p-orbital, while fluorine has a lone pair of electrons in its p-orbital. As a result, boron is a Lewis acid, while fluorine is a Lewis base. Back bonding is a form of bonding in which fluorine donates a lone pair of electrons to boron atoms. Boron trifluoride is a molecule made up of a Boron sp2 hybrid covalently bound to three fluorine atoms. The covalent bond indicates that electrons are exchanged rather than boron losing and fluorine gaining. Boron’s high ionization energy causes this bond to form. Three resonating structures result from the lone pair of electrons between boron and fluorine. Back bonding BF3 has no impact on the molecule’s bond angle, planarity, or geometry.The Boron trifluoride molecule has a ‘Trigonal Planar’ geometry. A model of three atoms around one atom in the centre is known as a ‘Trigonal Planar.’ It’s as if they’re all peripheral atoms in one plane, since the 120° bond angles on each of them make them an equilateral triangle.
0:00-1:14-Facts about Boron trifluoride(BF3)
1:14-3:10-Electron Deficiency of Boron trifluoride(BF3)
3:10-8:10--Reason for Back bonding in Boron trifluoride(BF3), weak Acidic Nature of Boron trifluoride(BF3),Multiple character in Boron trifluoride(BF3).
8:10-11:35-Resonance in Boron trifluoride(BF3),Resonating Structure of Boron trifluoride(BF3),Conclusion of back bonding in Boron trifluoride(BF3)
#NEET
#IITJEE
#JEEMAIN
#ConceptofbackbondinginBorontrluoride
#Hybridization
#Chemical Bonding for class 11
concept of back bonding in boron trifluoride: Boron has an empty p-orbital, while fluorine has a lone pair of electrons in its p-orbital. As a result, boron is a Lewis acid, while fluorine is a Lewis base. Back bonding is a form of bonding in which fluorine donates a lone pair of electrons to boron atoms. Boron trifluoride is a molecule made up of a Boron sp2 hybrid covalently bound to three fluorine atoms. The covalent bond indicates that electrons are exchanged rather than boron losing and fluorine gaining. Boron’s high ionization energy causes this bond to form. Three resonating structures result from the lone pair of electrons between boron and fluorine. Back bonding BF3 has no impact on the molecule’s bond angle, planarity, or geometry.The Boron trifluoride molecule has a ‘Trigonal Planar’ geometry. A model of three atoms around one atom in the centre is known as a ‘Trigonal Planar.’ It’s as if they’re all peripheral atoms in one plane, since the 120° bond angles on each of them make them an equilateral triangle.
0:00-1:14-Facts about Boron trifluoride(BF3)
1:14-3:10-Electron Deficiency of Boron trifluoride(BF3)
3:10-8:10--Reason for Back bonding in Boron trifluoride(BF3), weak Acidic Nature of Boron trifluoride(BF3),Multiple character in Boron trifluoride(BF3).
8:10-11:35-Resonance in Boron trifluoride(BF3),Resonating Structure of Boron trifluoride(BF3),Conclusion of back bonding in Boron trifluoride(BF3)
Комментарии
 0:11:35
0:11:35
 0:11:54
0:11:54
 0:10:30
0:10:30
 0:09:24
0:09:24
 0:58:39
0:58:39
 0:08:10
0:08:10
 0:10:24
0:10:24
 0:00:26
0:00:26
 0:00:14
0:00:14
 0:04:28
0:04:28
 0:00:51
0:00:51
 0:00:11
0:00:11
 0:00:16
0:00:16
 0:00:32
0:00:32
 0:00:06
0:00:06
 0:00:36
0:00:36
 0:02:34
0:02:34
 0:00:17
0:00:17
 0:44:12
0:44:12
 0:26:27
0:26:27
 0:00:36
0:00:36
 0:00:29
0:00:29
 0:00:46
0:00:46
 1:27:27
1:27:27