filmov
tv
Transition Metal Ion Hydroxide Precipitation Reactions (A-level Chemistry)
Показать описание
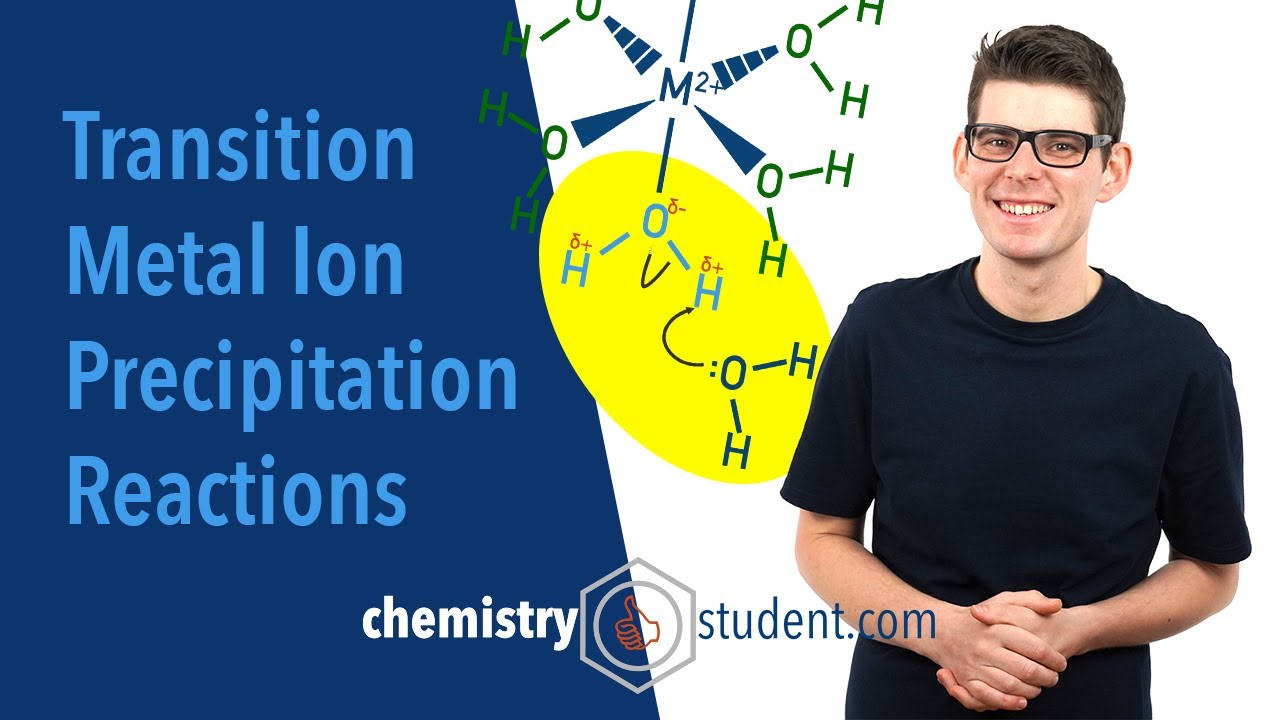
Outlining transition metal ion precipitation reactions with dropwise sodium hydroxide and ammonia to form metal hydroxides. The acid nature of metal aqua complex ions is explained and the amphoteric nature of aluminium hydroxide (Al(OH)3) and zinc hydroxide (Zn(OH)2). The use of precipitation reactions to identify transition metal ions is introduced and the effect of the charge to mass ratio of metal ions on the acidity of their metal aqua complex ions
For AQA, OCR (A), Edexcel and CIE.
Recap: 00:31
Metal Aqua Ion Precipitates: 02:10
EXAMPLE - Copper (II) Hydroxide: 03:57
Why are metal aqua complex ions acidic?: 05:07
Effect of adding OH- or NH3: 07:11
Factors that affect the acidities of aqua complex ions: 10:19
Amphoteric Hydroxides: 11:07
Summary: 12:21
Relevant Videos:
Thank you for watching - if you found the video useful, please like and subscribe!
For AQA, OCR (A), Edexcel and CIE.
Recap: 00:31
Metal Aqua Ion Precipitates: 02:10
EXAMPLE - Copper (II) Hydroxide: 03:57
Why are metal aqua complex ions acidic?: 05:07
Effect of adding OH- or NH3: 07:11
Factors that affect the acidities of aqua complex ions: 10:19
Amphoteric Hydroxides: 11:07
Summary: 12:21
Relevant Videos:
Thank you for watching - if you found the video useful, please like and subscribe!
 0:16:02
0:16:02
 0:08:46
0:08:46
 0:05:46
0:05:46
 0:01:14
0:01:14
 0:00:42
0:00:42
 0:04:06
0:04:06
 0:00:32
0:00:32
 0:03:04
0:03:04
 0:01:07
0:01:07
 0:01:02
0:01:02
 0:29:52
0:29:52
 0:03:43
0:03:43
 0:01:43
0:01:43
 0:09:36
0:09:36
 0:37:38
0:37:38
 0:12:33
0:12:33
 0:00:57
0:00:57
 0:09:01
0:09:01
 0:00:57
0:00:57
 0:09:35
0:09:35
 0:16:30
0:16:30
 0:00:50
0:00:50
 0:00:38
0:00:38
 0:04:00
0:04:00