filmov
tv
Rusting - Iron + water + oxygen = iron oxide
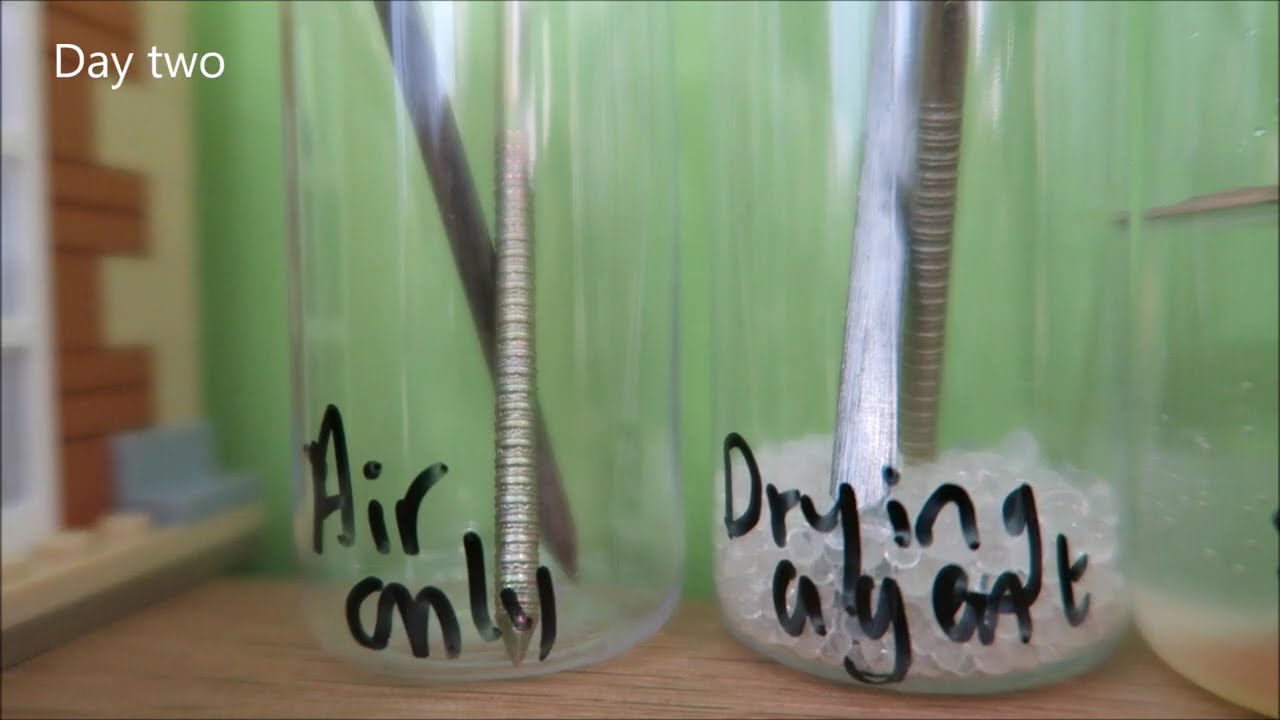
Показать описание
I want to help you achieve the grades you (and I) know you are capable of; these grades are the stepping stone to your future. Even if you don't want to study science or maths further, the grades you get now will open doors in the future.
Some of the links in here are affiliate links, where is get a small percentage of any money spent, if you like my channel and want to support my work, clicking these is an easy way to do it.
Disclaimer; You should not carry out any of these practical’s without carrying out a full risk assessment of your own first.
I am human, and I make mistakes, please point out any that you find and there is no need to follow that with a load of abuse.
PhET Interactive Simulations
University of Colorado Boulder
Music; Something Elated by Broke For Free. From the Free Music Archive, CC BY
Images from; Classroom Core (TpT), Hidesy Clipart (TpT), The Cher Room (TpT), The Triple Point (TpT), Ninja Woman (TpT), The Painted Crew (TpT) Teacher's Clipart (TpT) Shutterstock
Some of the links in here are affiliate links, where is get a small percentage of any money spent, if you like my channel and want to support my work, clicking these is an easy way to do it.
Disclaimer; You should not carry out any of these practical’s without carrying out a full risk assessment of your own first.
I am human, and I make mistakes, please point out any that you find and there is no need to follow that with a load of abuse.
PhET Interactive Simulations
University of Colorado Boulder
Music; Something Elated by Broke For Free. From the Free Music Archive, CC BY
Images from; Classroom Core (TpT), Hidesy Clipart (TpT), The Cher Room (TpT), The Triple Point (TpT), Ninja Woman (TpT), The Painted Crew (TpT) Teacher's Clipart (TpT) Shutterstock
Комментарии
 0:05:46
0:05:46
 0:04:58
0:04:58
 0:01:55
0:01:55
 0:03:11
0:03:11
 0:01:11
0:01:11
 0:00:58
0:00:58
 0:06:19
0:06:19
 0:02:47
0:02:47
 0:30:34
0:30:34
 0:00:40
0:00:40
 0:02:24
0:02:24
 0:01:01
0:01:01
 0:02:44
0:02:44
 0:07:00
0:07:00
 0:02:14
0:02:14
 0:00:28
0:00:28
 0:00:53
0:00:53
 0:01:00
0:01:00
 0:02:19
0:02:19
 0:10:41
0:10:41
 0:04:51
0:04:51
 0:09:06
0:09:06
 0:02:14
0:02:14
 0:00:24
0:00:24