filmov
tv
How to Find the Mass of One Atom of Lithium (Li)

Показать описание
There are two steps to find the mass of the Lithium (Li) atom. First we find the atomic mass of Li from the Periodic Table. We then divide this by Avogadro's Number (6.02 x E23).
Note that this is the mass of an average atom of Lithium since the periodic table lists the average atomic mass for elements. If you were given a specific isotope of Lithium you would divide the mass number for the isotope by Avogadro's number.
You should check your answer to make sure it makes sense. Since atoms are very, very small you should get a very small number as your answer.
Note that this is the mass for a single atom of Lithium (Li). In reality electronic balances are not able to measure the mass of one single atom at a time due to their small size.
For help with molar mass and chemical quantities, the following videos may be helpful:
Note that this is the mass of an average atom of Lithium since the periodic table lists the average atomic mass for elements. If you were given a specific isotope of Lithium you would divide the mass number for the isotope by Avogadro's number.
You should check your answer to make sure it makes sense. Since atoms are very, very small you should get a very small number as your answer.
Note that this is the mass for a single atom of Lithium (Li). In reality electronic balances are not able to measure the mass of one single atom at a time due to their small size.
For help with molar mass and chemical quantities, the following videos may be helpful:
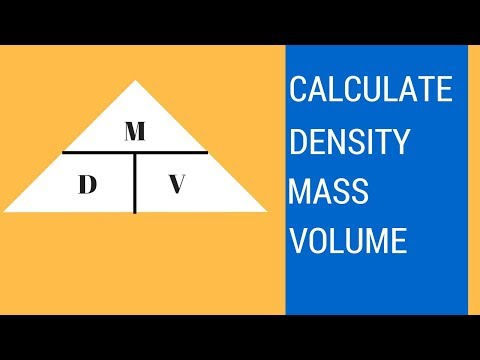
How to find density, mass, and volume
GCSE Chemistry - Relative Formula Mass
How To Calculate Relative Atomic Mass | Chemical Calculations | Chemistry | FuseSchool
How To Calculate The Molar Mass of a Compound - Quick & Easy!
Basic Calculations in Mathematics : How to Calculate Mass
Calculating masses in reactions - p27 (Chem)
Calculate the Mass of a Single Atom or Molecule
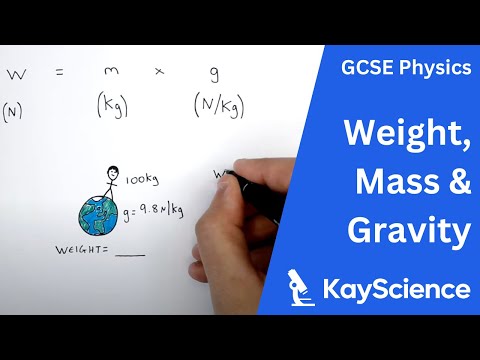
Weight, Mass, Gravity - (Weight = Mass x Gravitational Field Strength w=mxg) - GCSE Physics
O Rome Eternal | Holy Mass
How to Find the Mass of a Specific Element in a Compound
How To calculate Percentage Mass | Chemical Calculations | Chemistry | FuseSchool
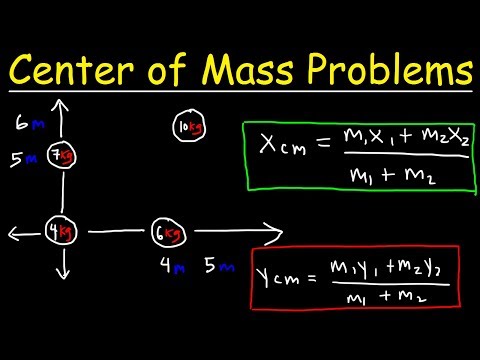
Center of Mass Physics Problems - Basic Introduction
Atomic Number, Mass Number, and Net Electric Charge
Density, Mass & Volume | GCSE Maths 2025
Mass Percent of a Solution Made Easy: How to Calculate Mass % or Make a Specific Concentration
Density, Mass and Volume relation? #math #tutor #physics #mathtrick #learning #shorts #youtube
Finding the Mass of a Metre Rule
How to Calculate Molar Mass (Molecular Weight)
Centre of Mass and Gravity GCSE Physics Required Practical
Trick to Calculate Atomic Mass of first 20 Elements #shorts #reels #chemistry
How to find centre of mass of an object
Centre of Mass | GCSE Physics | Doodle Science
How to Find the Center of Mass of (almost) Any 2D Shape | STEM Activity
What is amu || amu & gram || Class 9 chemistry
Комментарии
 0:05:17
0:05:17
 0:03:59
0:03:59
 0:03:48
0:03:48
 0:11:20
0:11:20
 0:01:25
0:01:25
 0:05:54
0:05:54
 0:07:16
0:07:16
 0:05:11
0:05:11
 0:01:06
0:01:06
 0:06:07
0:06:07
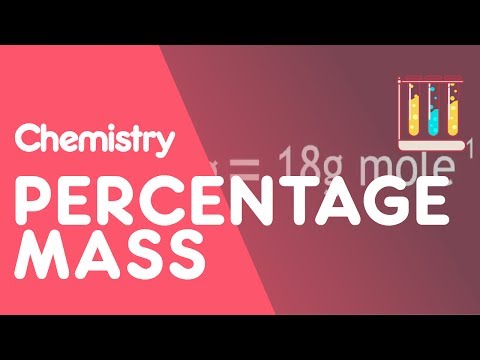 0:03:27
0:03:27
 0:13:14
0:13:14
 0:11:41
0:11:41
 0:00:56
0:00:56
 0:08:05
0:08:05
 0:00:30
0:00:30
 0:03:09
0:03:09
 0:03:51
0:03:51
 0:01:51
0:01:51
 0:00:54
0:00:54
 0:00:54
0:00:54
 0:01:51
0:01:51
 0:02:36
0:02:36
 0:00:20
0:00:20