filmov
tv
Polymers

Показать описание
Paul Andersen explains how polymers are formed from monomers. He describes how carbohydrates, protein and nucleic acids are created through condensation reactions. He also explains how these macromolecules are broken down through the process of hydrolysis.
Intro Music Atribution
Artist: CosmicD
Creative Commons Atribution License
Intro Music Atribution
Artist: CosmicD
Creative Commons Atribution License
GCSE Chemistry - What is a Polymer? Polymers / Monomers / Their Properties Explained #23
From DNA to Silly Putty: The diverse world of polymers - Jan Mattingly
Polymers: Crash Course Chemistry #45
Polymers - Basic Introduction
32. Polymers I (Intro to Solid-State Chemistry)
GCSE Chemistry - Condensation Polymers (Polyesters) #60
GCSE Chemistry - Addition Polymers & Polymerisation #56
Polymer Chemistry: Crash Course Organic Chemistry #35
Definition-Introduction-Classification of Polymers-Polymers-Chap 2- Unit 1 - NDDS 7th Sem B pharmacy...
Uses Of Polymers | Organic Chemistry | Chemistry | FuseSchool
Polymers - Condensation Polymerization
What is Polymerization?
V01_What is Polymer and the different Types of Polymers | understand the polymer in simple way
Polymerization Process -3D Animation / Polymerisationsprozess
Introduction to Polymers - Lecture 1.1. - What are polymers?
What is a Polymer?
Synthetic Polymers | Organic Chemistry | Chemistry | FuseSchool
AQA 3.12 Polymers REVISION
The Polymer Explosion: Crash Course Engineering #20
Addition Polymers Crash Course: HDPE, LDPE, PVC, Polystyrene and PTFE // HSC Chemistry
Polymer in One shot 🔥| 15 minutes Series| 4 marks guaranteed | NEET / JEE
33. Polymers II (Intro to Solid-State Chemistry)
Polymer Basics
Monomers and Polymers
Комментарии
 0:03:33
0:03:33
 0:05:00
0:05:00
 0:10:15
0:10:15
 0:26:19
0:26:19
 0:47:30
0:47:30
 0:05:19
0:05:19
 0:07:11
0:07:11
 0:13:15
0:13:15
 0:20:41
0:20:41
 0:03:53
0:03:53
 0:08:20
0:08:20
 0:01:45
0:01:45
 0:07:11
0:07:11
 0:03:34
0:03:34
 0:05:19
0:05:19
 0:01:45
0:01:45
 0:06:16
0:06:16
 0:36:04
0:36:04
 0:09:24
0:09:24
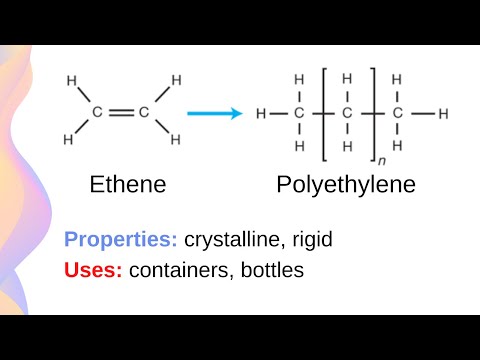 0:14:20
0:14:20
 0:18:14
0:18:14
 0:46:00
0:46:00
 0:23:38
0:23:38
 0:03:37
0:03:37