filmov
tv
Manganese Redox

Показать описание
ONE YEAR ANNIVERSARY OF STARTING THIS CHANNEL! :)
Thank you all so much for your interest in my hobby and your support into making it into something more.
NOTE: The "Hypomanganate" sample shown is actually likely an intermediate between the +7 and +6 states and not the actual +5 state.
Hypomanganate can only be made by very careful reduction of Manganate or permanganate below 10°C.
I may correct and reupload this video showing this if time allows.
Here is to many more years!
Synthesis all the oxidation states of manganese I could easily make in a home lab.
Note that the -2, -1. 0, and +1 states have also been isolated, but their difficulty of synthesis is prohibitive.
#chemistry #science #hydrogen #gas #elements #fire #chemical #industury #color
Thank you all so much for your interest in my hobby and your support into making it into something more.
NOTE: The "Hypomanganate" sample shown is actually likely an intermediate between the +7 and +6 states and not the actual +5 state.
Hypomanganate can only be made by very careful reduction of Manganate or permanganate below 10°C.
I may correct and reupload this video showing this if time allows.
Here is to many more years!
Synthesis all the oxidation states of manganese I could easily make in a home lab.
Note that the -2, -1. 0, and +1 states have also been isolated, but their difficulty of synthesis is prohibitive.
#chemistry #science #hydrogen #gas #elements #fire #chemical #industury #color
Manganese Redox
Redox rections: Manganese (KMnO4)
The oxidation states of manganese
redox reaction. #redox #manganese #oxide #manganese #chloride
Why Batteries work - Redox chemistry explained with zinc and manganese dioxide!
REDOX reaction between manganese and hydrogen peroxide #shorts #chemistry
Farmers need to release manganese and other metals from soil reserves. Here's how. | Regenerati...
How to Release Manganese and Other Metals from Soil Reserves
RedOx reactions- Class 11- L3(a)-Batch1&2- Oxidation number method balance, Q Link in descriptio...
Redox reaction | Reaction of Manganese Oxide with Hydrochloric acid | MnO2 + HCl Reaction | Class 10
REDOX reaction from permanganate to Mn(II) watch until the end!! #shorts #chemistry
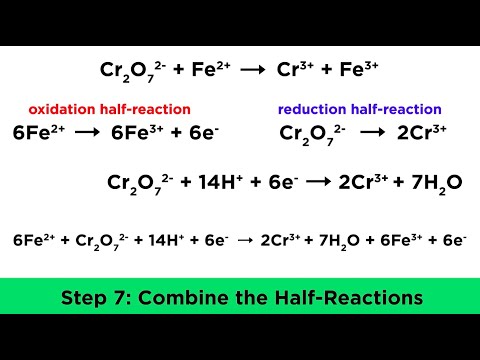
Redox Titration between MnO4- and Fe2+
Redox reaction between manganese and hydrogenperoxide #science #reactions #chemistry #viral #shorts
Balancing Redox Reactions in Acidic and Basic Conditions
Observations of different oxidation states of Mn containing compounds using redox reactions| No.22
Redox Reaction KMnO4 + H2O2 in RamZland!⚗️
balancing a redox reaction / oxidation number method
Lollipop lab Manganese ions (Redox)
The redox reactions of lollipops chupa chups.
How to find Oxidation Numbers for Manganese (Mn)
Quick demo - Redox - Potassium Manganate (VII) as an oxidising agent - CIE iGCSE Chemistry
Potassium manganate | Reducing Sugars & Redox Reaction
How to find the Oxidation Number for Mn in MnO2
Redox - oxidising and reducing agents
Комментарии
 0:05:40
0:05:40
 0:01:34
0:01:34
 0:00:42
0:00:42
 0:00:06
0:00:06
 0:05:37
0:05:37
 0:00:16
0:00:16
 1:06:25
1:06:25
 1:06:25
1:06:25
 0:32:47
0:32:47
 0:00:33
0:00:33
 0:00:16
0:00:16
 0:07:44
0:07:44
 0:00:16
0:00:16
 0:07:31
0:07:31
 0:06:28
0:06:28
 0:04:34
0:04:34
 0:03:35
0:03:35
 0:12:12
0:12:12
 0:00:59
0:00:59
 0:02:20
0:02:20
 0:02:59
0:02:59
 0:00:55
0:00:55
 0:01:05
0:01:05
 0:13:21
0:13:21