filmov
tv
Electron Energy Levels and Photons - IB Physics
Показать описание
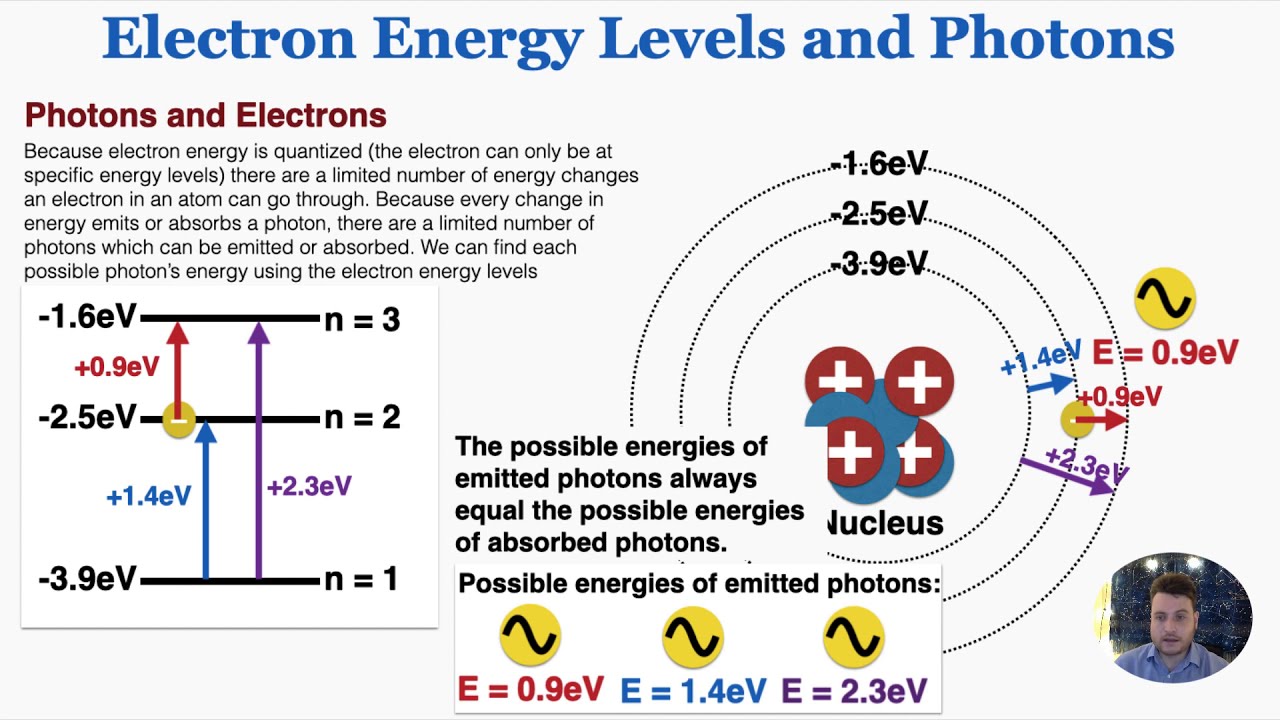
I explain electron energy levels, what it means for electron energy to be quantized, what must happen for an electron to jump to a different energy level, how to find the possible energies of photons emitted and absorbed by atoms based on their energy levels.
0:00 Negative Energy
2:06 Electrons and Negative Energy
4:23 Vocabulary
5:41 Quantized Energy
6:35 Notation for Energy Levels
7:14 Photons and Electron Energy
9:32 Finding Possible Photon Energy
12:36 Frequency and Wavelength
12:57 Example 1: Frequency and Wavelength
14:55 Example 2: Proportional Reasoning
16:38 Example 3: Absorbing a Photon
17:10 Example 4: Calculating Energy Levels
18:25 Extra Notes
0:00 Negative Energy
2:06 Electrons and Negative Energy
4:23 Vocabulary
5:41 Quantized Energy
6:35 Notation for Energy Levels
7:14 Photons and Electron Energy
9:32 Finding Possible Photon Energy
12:36 Frequency and Wavelength
12:57 Example 1: Frequency and Wavelength
14:55 Example 2: Proportional Reasoning
16:38 Example 3: Absorbing a Photon
17:10 Example 4: Calculating Energy Levels
18:25 Extra Notes
Electron Energy Levels and Photons - IB Physics
Energy Levels & Emission Spectra - A-level Physics
ABC Zoom - Electrons and photons: absorption and transmission of light
Electron Energy Levels - A Level Physics
Electron Energy and Light Spectra
Bohr Model of the Hydrogen Atom, Electron Transitions, Atomic Energy Levels, Lyman & Balmer Seri...
A Level Physics: Energy levels in atoms
Quantization of Energy Part 2: Photons, Electrons, and Wave-Particle Duality
Astronomy on Tap Seattle Livestream 10/12/24
Energy Levels and Photons
Where Do Electrons Get Their Everlasting Energy?
Electrons and Photons
Why Do Electrons Have Discrete Energy Levels?
Emission and Absorption Line Spectra - A Level Physics
Photons, Electron Energy Levels, Absorption Spectra
Y11-12 Physics: Line Spectra and Electron Energy Levels
22.3a Energy Levels and Line Spectra | A2 Quantum Physics | Cambridge A Level Physics
How To Calculate The Energy of a Photon Given Frequency & Wavelength in nm Chemistry
What are Energy Levels? Why? What is Quantum? How do Electrons Make Light Photons?
Bohr Model of the Hydrogen Atom
Energy levels of an atom ( IB Physics - Atomic Physics)
A Better Way To Picture Atoms
Emission and Absorption Line Spectra - A Level Physics
Photoelectric Effect - A-level Physics
Комментарии
 0:22:14
0:22:14
 0:13:39
0:13:39
 0:01:52
0:01:52
 0:04:55
0:04:55
 0:02:30
0:02:30
 0:21:44
0:21:44
 0:06:02
0:06:02
 0:05:40
0:05:40
 1:56:51
1:56:51
 0:04:42
0:04:42
 0:05:41
0:05:41
 0:01:16
0:01:16
 0:07:40
0:07:40
 0:05:12
0:05:12
 0:04:37
0:04:37
 0:09:21
0:09:21
 0:34:06
0:34:06
 0:11:06
0:11:06
 0:18:18
0:18:18
 0:04:50
0:04:50
 0:04:46
0:04:46
 0:05:35
0:05:35
 0:01:52
0:01:52
 0:09:39
0:09:39