filmov
tv
Plotting Liquid-Liquid Phase Equilibria (LLE), Part 2

Показать описание
Use liquid-liquid equilibria to break the binary azeotrope in an isopropanol-water system.
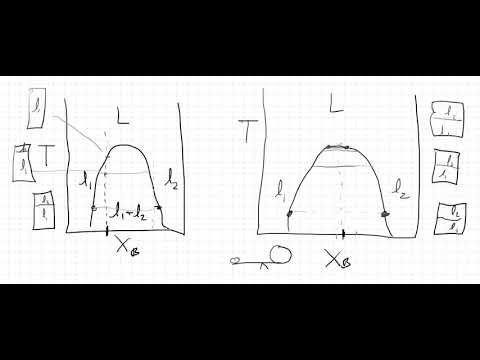
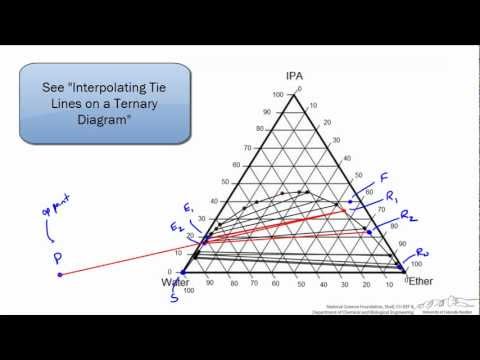
Select an appropriate solution model and the LLE global phase option, and ensure that the appropriate binary interaction parameters (BIPs) are present. Then create a binodal curve, using toluene as the first component, isopropanol as the second component, and water as the third component. (The first and third components exhibit the miscibility gap, and the second component distributes.)
The resulting plot shows that isopropanol prefers the toluene phase over the water phase, and illustrates the best way to use toluene to break the water-isopropanol azeotrope.
Select an appropriate solution model and the LLE global phase option, and ensure that the appropriate binary interaction parameters (BIPs) are present. Then create a binodal curve, using toluene as the first component, isopropanol as the second component, and water as the third component. (The first and third components exhibit the miscibility gap, and the second component distributes.)
The resulting plot shows that isopropanol prefers the toluene phase over the water phase, and illustrates the best way to use toluene to break the water-isopropanol azeotrope.
 0:06:14
0:06:14
 0:06:16
0:06:16
 0:11:51
0:11:51
 0:18:35
0:18:35
 0:02:47
0:02:47
 0:03:51
0:03:51
 0:09:02
0:09:02
 0:08:32
0:08:32
 0:50:11
0:50:11
 0:07:21
0:07:21
 0:25:06
0:25:06
 0:05:34
0:05:34
 0:06:42
0:06:42
 0:47:11
0:47:11
 0:05:02
0:05:02
 0:46:53
0:46:53
 0:06:31
0:06:31
 0:12:21
0:12:21
 0:07:13
0:07:13
 1:34:45
1:34:45
 0:05:15
0:05:15
 0:03:08
0:03:08
 1:27:20
1:27:20
 0:44:33
0:44:33