filmov
tv
Resonance Structures of NO3(-1), nitrate ion

Показать описание
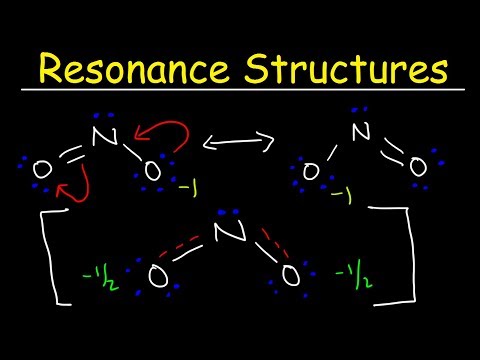
There are three equally-valid Lewis structures for the nitrate ion, which is one nitrogen atom surrounded by three oxygen atoms (with one bonus electron from the -1 charge). These combine to make a "resonance hybrid" which reflects the delocalized electrons among the three N-O bonds.
Resonance Structures of NO3(-1), nitrate ion
Resonance Structures for NO3- (Nitrate Ion)
How To Draw The Lewis Structure of NO3- (Nitrate Ion)
Resonance: The resonance structures of the nitrate (NO3(-)) ion
Resonance structures of nitrate and acetate ions
Resonance Structures, Basic Introduction - How To Draw The Resonance Hybrid, Chemistry
Resonance Structures Example
Bonding 32: The Resonance Structures of the Nitrate Ion
Resonance Structure of NO3- (nitrate ion)
Lewis and Hybrid Structure of Nitrate ion [NO₃⁻]
Resonance structures of NO3 - ( Nitrate ion)
Sketch the resonance structures for the nitrate ion NO3- Is the hybridization of the N atom the s
Resonance structures of nitrate ion/ resonance hybrid structure of nitrate ion, by khushboo yadav
How to Draw the Lewis Dot Structure for NO3 - (Nitrate ion)
Resonance and Formal Charges: Nitrate Ion
resonance: nitrate and phosphate
How many resonance forms can be written for the nitrate ion, \( \left(\mathrm{NO}_{3}^{-}\right)...
'Examples of Resonance' [ nitrate ion]
Resonance and dot structures | Chemical bonds | Chemistry | Khan Academy
How Many Resonance Structures Are Possible for the Nitrate Ion?
Resonance and Dot Structures
Nitrate Ion Lewis Structure: How to Draw the Lewis Structure for Nitrate Ion
Drawing Lewis Structures: Nitrate Ion
Bonding 78: Drawing Resonance Structures #1 Nitrate Ion
Комментарии
 0:05:32
0:05:32
 0:01:28
0:01:28
 0:05:52
0:05:52
 0:09:55
0:09:55
 0:15:43
0:15:43
 0:10:31
0:10:31
 0:04:13
0:04:13
 0:07:05
0:07:05
 0:02:37
0:02:37
 0:07:58
0:07:58
 0:08:35
0:08:35
 0:10:54
0:10:54
 0:02:45
0:02:45
 0:04:07
0:04:07
 0:13:29
0:13:29
 0:05:24
0:05:24
 0:01:29
0:01:29
 0:04:30
0:04:30
 0:06:15
0:06:15
 0:03:27
0:03:27
 0:06:13
0:06:13
 0:01:27
0:01:27
 0:03:45
0:03:45
 0:10:32
0:10:32