filmov
tv
De broglie equation / wave nature of matter / structure of atom - class 11

Показать описание
DE BROGLIE DERIVATION
De Broglie's equation may be derived by combining mass energy relationships proposed by Max Planck and Einstein. According to Planck's quantum theory, photon is assumed to have wave character and its energy is given by
E=hv ........ (1)
Where v = frequency of the wave, h = Plank's constant.
According to Einstein equation, photon is supposed to have particle character and its energy is given by
E=mc2 ........ (2)
where m = mass of photon, c = velocity of light.
From equations (1) and (2), we get,
hv=mc2
But v=λc wavelengthvelocity=frequency
∴λhc=mc2
λ=mch λ=mch
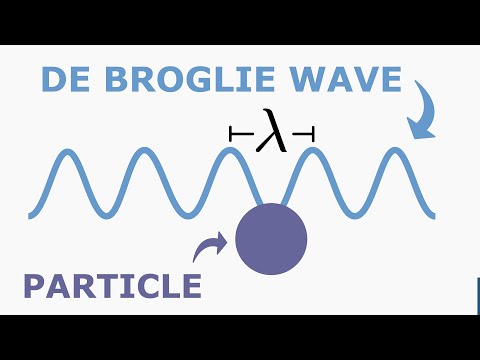
The above equation is applicable to material particle like electron. We may write
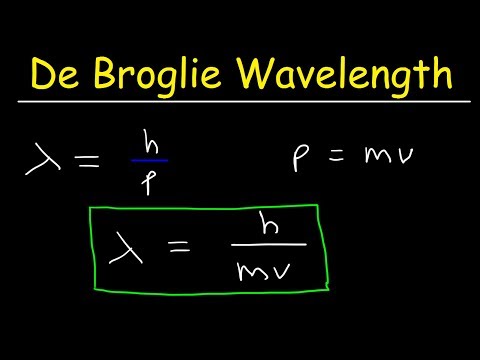
λ=mvh or λ=ph
where mv = p is the momentum of the particle.
λ=ph is called de Broglie equation and 'λ' = de Broglie wavelength.
Q.how to calculated mass of photon by de broglie equation?
De Broglie's equation may be derived by combining mass energy relationships proposed by Max Planck and Einstein. According to Planck's quantum theory, photon is assumed to have wave character and its energy is given by
E=hv ........ (1)
Where v = frequency of the wave, h = Plank's constant.
According to Einstein equation, photon is supposed to have particle character and its energy is given by
E=mc2 ........ (2)
where m = mass of photon, c = velocity of light.
From equations (1) and (2), we get,
hv=mc2
But v=λc wavelengthvelocity=frequency
∴λhc=mc2
λ=mch λ=mch
The above equation is applicable to material particle like electron. We may write
λ=mvh or λ=ph
where mv = p is the momentum of the particle.
λ=ph is called de Broglie equation and 'λ' = de Broglie wavelength.
Q.how to calculated mass of photon by de broglie equation?
Derive De-Broglie Wave Equation | Quantum Chemistry | Physical Chemistry
De Broglie wavelength | Physics | Khan Academy
De-Broglie Wavelength
De Broglie equation
The de Broglie Wavelength and Wave Particle Duality - A Level Physics
De Broglie Hypothesis | De Broglie Wavelength
De Broglie Wavelength Problems In Chemistry
Derivation of de Broglie Relationship | Atomic Structure | Chemistry |Chapter 2 | NEET | JEE
Bohr Atomic Model | Dual nature of matter - de Broglie | Heisenberg Uncertainty Principle | JEE NEET
de Broglie’s proposal
De Broglie Hypothesis | De Broglie Wavelength
Deriving The De Broglie Wavelength
Derivation of De Broglie Equation | De Broglie Wavelength #physics #cbse #neet #jeemains
The de Broglie Equation and Why There Is No Wave-Particle Duality
Wave|Nature|Matter|De Broglie|Wavelength|Physics 12|Tamil|MurugaMP
Your Daily Equation #9: De Broglie Wavelength
de broglie's equation♥️🔥|Atomic structure| |chemistry|
De broglie equation / wave nature of matter / structure of atom - class 11
Derivation of De Broglie Equation|De Broglie wavelength #physics #cbse #iitjee #jeemains
Atomic structure || de Broglie Equations
Wave-particle duality and the de Broglie equation
De broglie equation, Dual nature of matter,
derivation of de broglie equation
The De Broglie Equation Explained!
Комментарии
 0:01:43
0:01:43
 0:11:20
0:11:20
 0:05:15
0:05:15
 0:01:00
0:01:00
 0:04:29
0:04:29
 0:09:05
0:09:05
 0:11:21
0:11:21
 0:00:06
0:00:06
 1:46:32
1:46:32
 0:10:37
0:10:37
 0:19:00
0:19:00
 0:03:41
0:03:41
 0:00:56
0:00:56
 0:11:53
0:11:53
 0:17:25
0:17:25
 0:21:29
0:21:29
 0:00:05
0:00:05
 0:09:55
0:09:55
 0:00:52
0:00:52
 0:00:13
0:00:13
 0:14:31
0:14:31
 0:00:07
0:00:07
 0:02:11
0:02:11
 0:03:02
0:03:02