filmov
tv
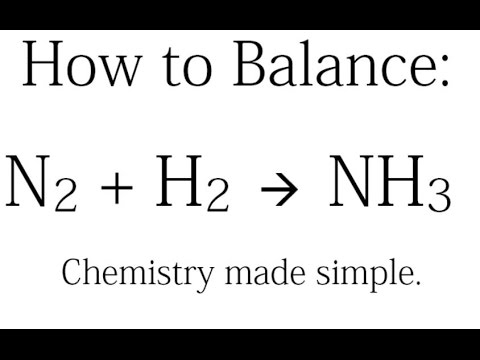
If the synthesis of ammonia from Haber's process is carried out with exactly the same starting co...

Показать описание
If the synthesis of ammonia from Haber's process is carried out with exactly the same starting conditions (of partial pressure and temperature) but using D_2 (deuterium) in place of H_2. Then
(a) the equilibrium will be disturbed
(b) the composition of reaction mixture will remain same at equilibrium.
(c) Use of isotope in reaction will not produce ammonia.
(d) At equilibrium rate of forward reaction will be greater than the rate of reverse reaction
📌 PHYSICS WALLAH OTHER CHANNELS :
📌 PHYSICS WALLAH SOCIAL MEDIA PROFILES :
(a) the equilibrium will be disturbed
(b) the composition of reaction mixture will remain same at equilibrium.
(c) Use of isotope in reaction will not produce ammonia.
(d) At equilibrium rate of forward reaction will be greater than the rate of reverse reaction
📌 PHYSICS WALLAH OTHER CHANNELS :
📌 PHYSICS WALLAH SOCIAL MEDIA PROFILES :
 0:03:06
0:03:06
 0:04:05
0:04:05
 0:01:00
0:01:00
 0:07:19
0:07:19
 0:02:37
0:02:37
 0:02:13
0:02:13
 0:38:44
0:38:44
 0:32:55
0:32:55
 0:01:16
0:01:16
 0:02:48
0:02:48
 0:10:02
0:10:02
 0:03:04
0:03:04
 0:03:18
0:03:18
 0:21:34
0:21:34
 0:07:04
0:07:04
 0:05:19
0:05:19
 0:09:52
0:09:52
 0:36:19
0:36:19
 0:07:54
0:07:54
 0:10:19
0:10:19
 0:01:58
0:01:58
 0:22:16
0:22:16
 0:11:28
0:11:28
 0:12:59
0:12:59