filmov
tv
Why Are Covalent Bonds Strong - GCSE Chemistry | kayscience.com

Показать описание
In this video you will learn all the science for this topic to get a grade 9 or A* in your science exams!
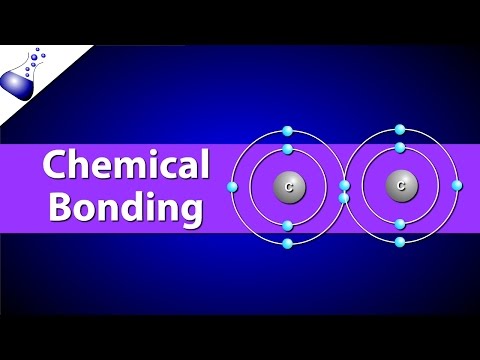
A covalent bond is the shared pair of electrons between non-metal atoms. Non-metal atoms are found to the right of the stepped line in the periodic table. Non-metal atoms share their outer electrons to have a full outer shell of electrons forming stable molecules. Molecules have a neutral charge as their atoms have the same number of protons and electrons which cancel each other out.
Covalent bonds are strong because there is a strong electrostatic force of attraction between the positive protons in the nucleus and the shared pair of negative electrons, which requires a lot of energy to break.
A covalent bond is the shared pair of electrons between non-metal atoms. Non-metal atoms are found to the right of the stepped line in the periodic table. Non-metal atoms share their outer electrons to have a full outer shell of electrons forming stable molecules. Molecules have a neutral charge as their atoms have the same number of protons and electrons which cancel each other out.
Covalent bonds are strong because there is a strong electrostatic force of attraction between the positive protons in the nucleus and the shared pair of negative electrons, which requires a lot of energy to break.
Комментарии