filmov
tv
Empirical Formula from Experimental Data
Показать описание
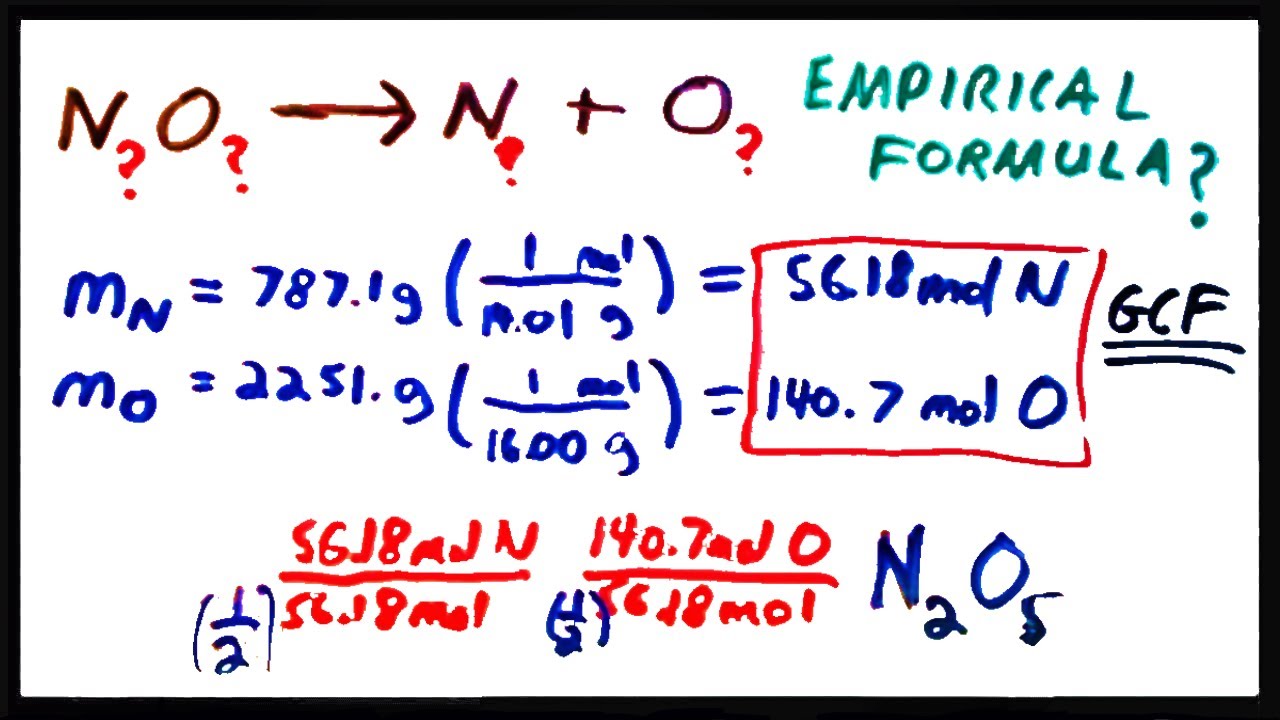
How to calculate the empirical formula of a compound. All you need to do is find some way to decompose the compound into its constituent elements, measure the masses of those elements, convert those masses into moles, and then find the greatest common factor of the molar amounts of the elements.
Thanks for watching! 😀
Follow me on:
#EmpiricalFormula #ChemicalFormula #Chemistry #GeneralChemistry #Decomposition #Mass #Mole #Moles #MolarMass #PeriodicTable #BensChemVideos #Element #Elements #Compound #Compounds
Thanks for watching! 😀
Follow me on:
#EmpiricalFormula #ChemicalFormula #Chemistry #GeneralChemistry #Decomposition #Mass #Mole #Moles #MolarMass #PeriodicTable #BensChemVideos #Element #Elements #Compound #Compounds
Empirical Formula from Experimental Data
1.2 Calculate empirical formula from experimental data
OCR AS Chemistry - Calculating Empirical Formula From Experimental Data 1
Empirical formula from experimental data
Calculating Empirical Formula From Experimental Data
Empirical formula from experimental data
Empirical Formula from Experimental Data
Determining empirical formulas from experimental data - Real Chemistry
Beyond CMOS technology and advanced nano electronics with Nanodcal S/W
Finding Empirical Formulas from Experimental Data
Empirical Formula & Molecular Formula Determination From Percent Composition
⚗️ Obtaining an Empirical Formula from Experimental Data (Part 1)
CHEMISTRY 101: Determining molecular formula from experimental data
Emperical Formula from Experiment
Calculating Empirical Formulas from Experimental Data
Determining empirical and molecular formulas from experimental data
3.6 Determination of Empirical Formula
1.2.6 Determine the molecular formula when given both the empirical formula and experimental data.
Calculating Percent Mass and Formulas from Experimental Data
CHEM 139 Chapter 8 V7 Empirical Formula from Experimental Data
1.2.6 Determine the molecular formula when given both the empirical formula and experimental data.
OCR AS Chemistry - Calculating the Empirical Formula from Experimental Data 2 (One Product)
Finding and Calculating an Empirical Formula of a Compound | How to Pass Chemistry
Determining a Chemical Formula from Experimental Data
Комментарии
 0:07:57
0:07:57
 0:03:17
0:03:17
 0:08:58
0:08:58
 0:03:52
0:03:52
 0:04:22
0:04:22
 0:03:52
0:03:52
 0:07:56
0:07:56
 0:16:11
0:16:11
 1:36:15
1:36:15
 0:09:36
0:09:36
 0:11:00
0:11:00
 0:06:27
0:06:27
 0:04:59
0:04:59
 0:09:12
0:09:12
 0:10:56
0:10:56
 0:16:30
0:16:30
 0:11:06
0:11:06
 0:01:46
0:01:46
 0:03:10
0:03:10
 0:14:40
0:14:40
 0:00:55
0:00:55
 0:07:33
0:07:33
 0:02:52
0:02:52
 0:15:48
0:15:48