filmov
tv
ChemHelp / Drawing a Lewis dot diagram for hydroxide, OH-

Показать описание
ChemHelp / Drawing a Lewis dot diagram for hydroxide, OH-
ChemHelp / Drawing a Lewis dot diagram for KBr
ChemHelp / Drawing a Lewis dot diagram for AlF3
ChemHelp / Drawing a Lewis dot diagram for PCl3
ChemHelp / Drawing a Lewis dot diagram for methane, CH4
ChemHelp / Drawing a Lewis dot diagram for acetylene, C2H2
ChemHelp / Drawing a Lewis dot diagram for Na2O
4.5 - Drawing Lewis Dot Diagrams
ChemHelp Drawing a Lewis dot diagram for carbonate, CO32
drawing Lewis dot structures
How to Draw a Lewis Structure From a Molecular Formula C6H14O2 Help with Chemistry!
Introducing the Basic Lewis Structure Playlist!
How to Draw a Lewis Structure From a Molecular Formula C5H8Cl2 Help with Chemistry!
Math Method for Drawing Lewis Structures
How to Draw the Lewis Dot Structure for the Hydroxide ion
converting the line-angle structure of acetaminophen to Lewis dot
How to Calculate Formal Charge | Formal Charge Calculation in Lewis Structures | AP chemistry
Live Stream- Lewis Structures
27.1.2 - Drawing Structural Formulas
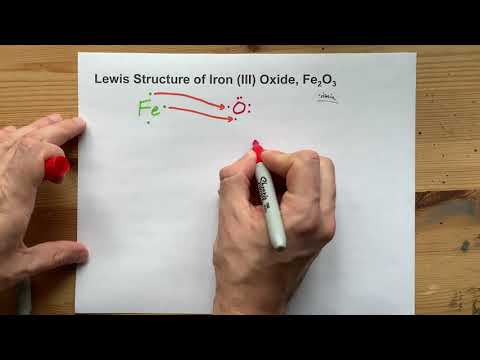
Lewis Structure of Fe2O3, Iron (III) Oxide
Live Stream- Lewis Structures
converting line-angle to Lewis dot structures
styles in Lewis dot structures
5.3 - Drawing Orbital Diagrams
Комментарии
 0:02:10
0:02:10
 0:01:43
0:01:43
 0:02:20
0:02:20
 0:02:27
0:02:27
 0:02:14
0:02:14
 0:02:04
0:02:04
 0:01:41
0:01:41
 0:26:15
0:26:15
 0:03:25
0:03:25
 0:04:30
0:04:30
 0:05:59
0:05:59
 0:00:18
0:00:18
 0:06:08
0:06:08
 0:06:51
0:06:51
 0:01:59
0:01:59
 0:03:10
0:03:10
 0:07:32
0:07:32
 1:05:34
1:05:34
 0:27:23
0:27:23
 0:04:01
0:04:01
 1:06:52
1:06:52
 0:14:22
0:14:22
 0:02:39
0:02:39
 0:17:38
0:17:38