filmov
tv
Percent Ionization - Example 1

Показать описание
In this video, I solve a percent ionization problem. It involves the pH, the pOH, and the equilibrium constant for a base.
The reaction is:
NH3 «-----» NH4+ + OH-
Problem:
It is found that 0.010M of NH3 has undergone 4.2% ionization. Find the pH value and the Kb value. Is the solution acidic, basic, or neutral?
=================================================
Thanks for Watching! I hope I was able to guide you towards your success!
Like! Share! Subscribe! Comment!
=================================================
The reaction is:
NH3 «-----» NH4+ + OH-
Problem:
It is found that 0.010M of NH3 has undergone 4.2% ionization. Find the pH value and the Kb value. Is the solution acidic, basic, or neutral?
=================================================
Thanks for Watching! I hope I was able to guide you towards your success!
Like! Share! Subscribe! Comment!
=================================================
Percent Ionization - Example 1
Calculate the Percent Ionization of 0.65 M HNO2
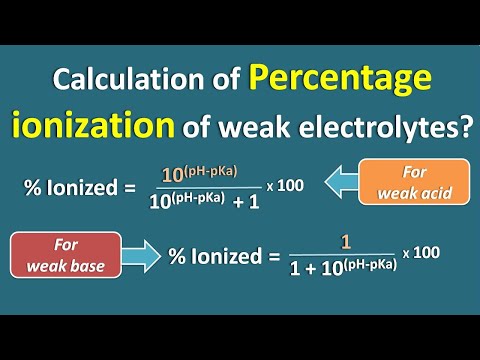
Calculation of percent ionized of weak electrolytes
pH of Weak Acids and Bases - Percent Ionization - Ka & Kb
Worked example: Finding the percent ionization of a weak acid | AP Chemistry | Khan Academy
General Chemistry | Percent Ionization Calculation
How to use percent ionization to find Ka
Percent ionization
Finding the Percent Ionization of a Weak Acid-Practice Problems
Percent Ionization
Percent Ionized Calculations
Percent Ionization of a Weak Acid
Percent Ionization
Weak Acid Ionization (Degree of Ionization & Percent Ionization for Ex 1 & Ex 2)
CH14Q14 percent ionization from ka
⚗️ Finding the Percent Ionization of a Weak Acid
Measure weak acid—percent ionization
Percent ionization of a weak acid
pH and percent ionization of strong and weak bases
pH and Percent Ionization of Weak Acid Example Problems
Percent Ionization
Common ion effect on percent ionization
What is percent ionization
Ph and Pka,, percent ionization
Комментарии
 0:04:10
0:04:10
 0:04:57
0:04:57
 0:10:23
0:10:23
 0:29:31
0:29:31
 0:06:50
0:06:50
 0:04:38
0:04:38
 0:03:52
0:03:52
 0:03:44
0:03:44
 0:14:23
0:14:23
 0:06:12
0:06:12
 0:06:20
0:06:20
 0:16:32
0:16:32
 0:11:02
0:11:02
 0:09:52
0:09:52
 0:04:19
0:04:19
 0:07:35
0:07:35
 0:04:28
0:04:28
 0:09:21
0:09:21
 0:34:02
0:34:02
 0:22:00
0:22:00
 0:03:39
0:03:39
 0:07:57
0:07:57
 0:08:05
0:08:05
 0:09:28
0:09:28