filmov
tv
Make Hydrochloric Acid
Показать описание
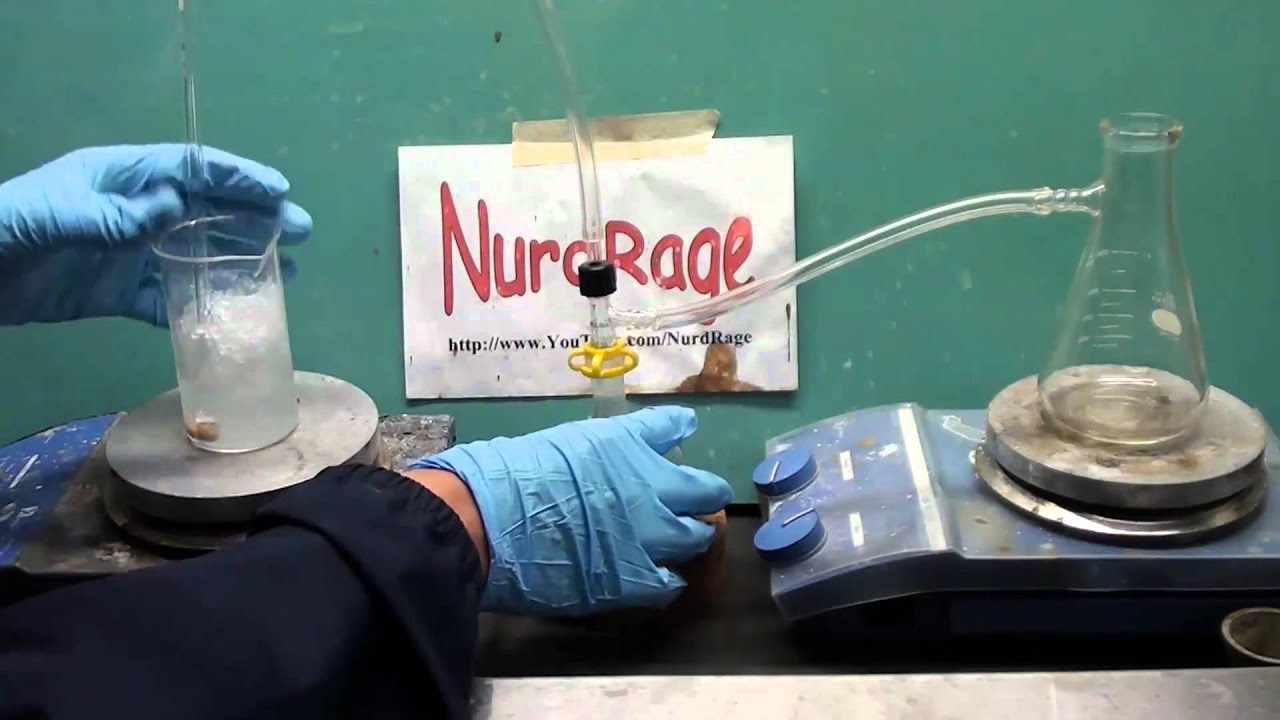
We show how to make hydrochloric acid from sodium bisulfate and table salt.
The synthesis is rather simple, we generate hydrogen chloride gas by mixing together 140g of sodium bisulfate and 60g of sodium chloride salt and then heating. 20mL of water can be added to lower the temperature required but this is optional. Hydrogen chloride gas will be produced. This gas is lead into distilled water to produce hydrochloric acid. Be careful though, hydrogen chloride is so soluble that the water will pull itself into the tube trying to absorb it. In the video i use an elaborate back-flow prevention system to prevent loss of the product.
Sodium bisulfate is available as a pH lowering chemical. 30mL of sulfuric acid may be used as a replacement if you have it. Although if you had that, you probably don't need hydrochloric acid.
The synthesis is rather simple, we generate hydrogen chloride gas by mixing together 140g of sodium bisulfate and 60g of sodium chloride salt and then heating. 20mL of water can be added to lower the temperature required but this is optional. Hydrogen chloride gas will be produced. This gas is lead into distilled water to produce hydrochloric acid. Be careful though, hydrogen chloride is so soluble that the water will pull itself into the tube trying to absorb it. In the video i use an elaborate back-flow prevention system to prevent loss of the product.
Sodium bisulfate is available as a pH lowering chemical. 30mL of sulfuric acid may be used as a replacement if you have it. Although if you had that, you probably don't need hydrochloric acid.
Make Hydrochloric Acid
Turning salt into hydrochloric acid
Make Hydrochloric Acid
How to Make Hydrochloric Acid (HCl) at Home - 1st method: Reaction of H2SO4 with NaCl
How to make HCl at home | Household materials
Making Hydrochloric Acid (Old Version)
How to make Hydrochloric Acid at Home With Salt & Sulfuric Acid
Making Hydrochloric acid
Concentrated Hydrochloric acid 🥼🧪🧫💥💀⚗️ #science #diy #instagram #yt #chemistry #chemical #cocomelon...
How to Concentrate HCl (Azeotropic Distillation)
Hydrochloric Acid Lemonade
Preparation of Hydrochloric Acid ( HCl )
How to make hydrochloric acid
Turning Table Salt into Acid (All About Acids)
Why Can’t My Body Make Hydrochloric Acid HCl
How to make HCl and NaOH at Home by using Salt | Making HCl & NaOH at home|#chemicals#salt#trend...
How to Make Hydrochloric Acid at Home In Hindi || Make hydrochloric Acid from Table Salt in Hindi
Make HCl and NaOH at home with salt - EP 4
How to Make a 0.1M HCl Solution (Hydrochloric acid)
How To Make Hcl Acid At Home.Hcl Acid is Very Danger Eliment Earth.Hydrochloric Acid.
Making HCl out of H2SO4 and NaCl
How to make hydrochloric acid with vinegar and salt?
Turning Vinegar into Acetic Acid using stuff from Walmart
Making Sulphuric acid (Easiest way)
Комментарии
 0:03:04
0:03:04
 0:07:51
0:07:51
 0:02:23
0:02:23
 0:10:14
0:10:14
 0:09:30
0:09:30
 0:02:20
0:02:20
 0:03:46
0:03:46
 0:04:31
0:04:31
 0:00:07
0:00:07
 0:03:32
0:03:32
 0:03:34
0:03:34
 0:02:54
0:02:54
 0:03:13
0:03:13
 0:15:16
0:15:16
 0:11:22
0:11:22
 0:08:37
0:08:37
 0:12:20
0:12:20
 0:16:23
0:16:23
 0:02:15
0:02:15
 0:01:18
0:01:18
 0:03:20
0:03:20
 0:01:19
0:01:19
 0:04:16
0:04:16
 0:04:21
0:04:21