filmov
tv
How lead acid battery works | Working principle animation

Показать описание
Hi everyone!!
In Electric vehicles, one of the most widely used battery is lead acid battery.
In this video let us understand how lead acid battery works.
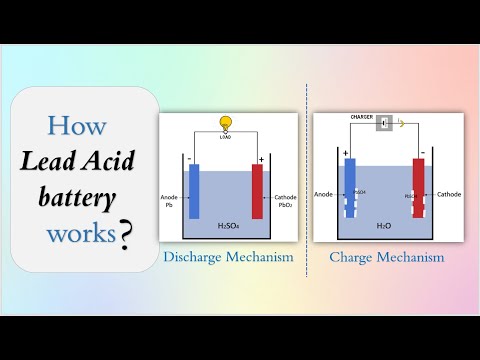
The basic components of lead acid battery, the anode is made of spongy lead (Pb). The cathode is made of lead peroxide (PbO2). The electrolyte is diluted Sulfuric acid.
The separator is made of Absorbent Glass Mat (AGM).
The charging and discharging mechanism is explained along with the cell reaction at cathode and anode.
Watch the video to find out more.
Like, Share and Subscribe to the channel for more videos and content coming up!
.
.
.
In Electric vehicles, one of the most widely used battery is lead acid battery.
In this video let us understand how lead acid battery works.
The basic components of lead acid battery, the anode is made of spongy lead (Pb). The cathode is made of lead peroxide (PbO2). The electrolyte is diluted Sulfuric acid.
The separator is made of Absorbent Glass Mat (AGM).
The charging and discharging mechanism is explained along with the cell reaction at cathode and anode.
Watch the video to find out more.
Like, Share and Subscribe to the channel for more videos and content coming up!
.
.
.
How Lead Acid Batteries Work: A Simple Guide
How lead acid battery works | Working principle animation
How a lead-acid battery works
How A Car Battery Works - basic working principle
How lead acid battery works. ✔
Lead Acid Battery: How does it work? | Hawker Powersource, Inc.
How a lead acid battery works
How the Lead-Acid Battery Works
HAWKMOTO 36V 1000W Electric Dirt Bike / Upgraded Lithium Baterry From x3 12V Lead Acids !
Working Principle of Lead Acid Battery
ALTERNATORS & BATTERIES | How They Work
Brilliant technique of lead acid battery restoration
Flooded Lead Acid Battery: How does it work? | Hawker Powersource, Inc.
What Makes a Deep Cycle Battery Different
Battery 101: The Fundamentals of How A Lithium-Ion Battery Works
Principle Of Lead Acid Battery || How it Works || 3D Animation
Lead – Acid cell
Lead Acid Battery Construction
Lead Acid Battery Charging Stages
Inside view of lead acid battery before and after desulfator
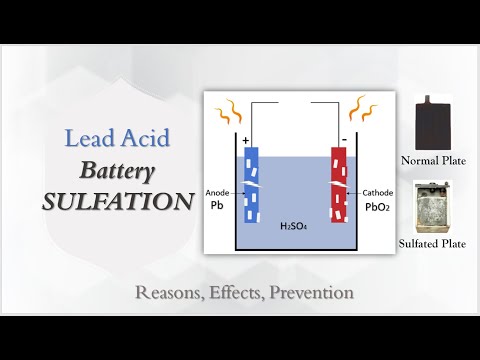
Lead Acid Battery Sulfation | Battery Sulfation
Manufacture and Assembly of Lead Acid Battery
Working of Rechargeable Battery #vigyanrecharge
How do Lead Acid Batteries Work? | SKILL-LYNC
Комментарии
 0:04:57
0:04:57
 0:03:57
0:03:57
 0:04:56
0:04:56
 0:16:01
0:16:01
 0:04:35
0:04:35
 0:02:06
0:02:06
 0:13:17
0:13:17
 0:03:59
0:03:59
 0:10:46
0:10:46
 0:04:53
0:04:53
 0:07:18
0:07:18
 0:11:23
0:11:23
 0:02:10
0:02:10
 0:09:53
0:09:53
 0:04:48
0:04:48
 0:03:26
0:03:26
 0:02:59
0:02:59
 0:13:17
0:13:17
 0:04:08
0:04:08
 0:02:11
0:02:11
 0:03:06
0:03:06
 0:02:40
0:02:40
 0:09:52
0:09:52
 0:03:50
0:03:50