filmov
tv
What is Osmosis? - Part 1 | Cell | Infinity Learn

Показать описание
But how does this process work? What is osmosis? Why does water only enters the cells? Can it travel in the other directions outside the cell as well? Watch this video to get introduced to this interesting concept of "Osmosis".
In this video, we will learn:
0:00 Introduction
0:31 active transport
1:19 diffusion - passive transport
1:40 Osmosis
NCERT Solutions for Class 6 to 12 – Free CBSE NCERT Solutions
Register on our website to gain access to all videos and quizzes:
#Cell #Osmosis #Biology #neet2024 #infinityLearnNEET #neetsyllabus #neet2025
In this video, we will learn:
0:00 Introduction
0:31 active transport
1:19 diffusion - passive transport
1:40 Osmosis
NCERT Solutions for Class 6 to 12 – Free CBSE NCERT Solutions
Register on our website to gain access to all videos and quizzes:
#Cell #Osmosis #Biology #neet2024 #infinityLearnNEET #neetsyllabus #neet2025
What Is Osmosis? | The Dr. Binocs Show | Best Learning Videos For Kids | Peekaboo Kidz
Osmosis | Membrane Transport
What is Osmosis? - Part 1 | Cell | Infinity Learn
Transport in Cells: Diffusion and Osmosis | Cells | Biology | FuseSchool
Diffusion, Osmosis and Dialysis (IQOG-CSIC)
Osmosis Process - Part 2 | Don't Memorise
Biology - What is Osmosis - Plasma Membrane - Part 1 - English
Diffusion And Osmosis | Cell-Structure & Function | Biology | Class 9
2024 GCE BIOLOGY PAPER 2 (SECTION A) Q1
Transport In Cells: Active Transport | Cells | Biology | FuseSchool
GCSE Biology - Osmosis #8
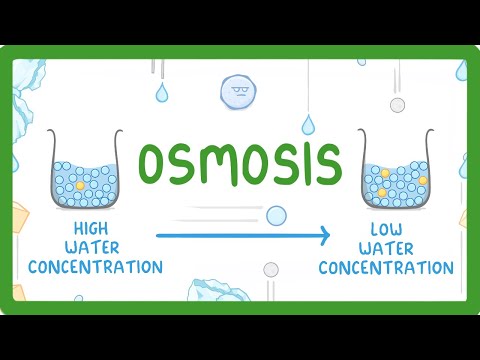
Osmosis
Osmosis Vs Diffusion | Concept Of Passive transport |
SAVE MY EXAMS | What is OSMOSIS? (Part 1)
Diffusion and Osmosis
What is osmosis? | osmosis and it's type | osmosis | importance of osmosis
What is Osmosis || Class 12 || Chemistry || Alakh Pandey Sir || @AlakhSirHighlights
IV Fluids & Osmosis Part I - Nursing Theory
What is osmosis process | Hypertonic, hypotonic and isotonic solutions
Osmosis - GCSE Biology (9-1)
Osmosis revision part 1: Describing osmosis
Osmosis and Osmotic Pressure || 3D Animated Explanation || class 12th chemistry || Solutions ||
Osmosis #shorts
Basics of osmosis #biology
Комментарии
 0:07:06
0:07:06
 0:06:02
0:06:02
 0:02:59
0:02:59
 0:03:52
0:03:52
 0:01:36
0:01:36
 0:03:32
0:03:32
 0:06:08
0:06:08
 0:07:44
0:07:44
 0:03:41
0:03:41
 0:02:32
0:02:32
 0:04:24
0:04:24
 0:00:41
0:00:41
 0:10:31
0:10:31
 0:00:56
0:00:56
 0:09:30
0:09:30
 0:02:54
0:02:54
 0:03:42
0:03:42
 0:07:17
0:07:17
 0:06:21
0:06:21
 0:07:41
0:07:41
 0:13:33
0:13:33
 0:03:12
0:03:12
 0:00:46
0:00:46
 0:00:53
0:00:53