filmov
tv
Making H2SO4 from Sulfates and HCl

Показать описание
In this video, I'll show you a quite unique way of making sulphuric acid, from the weaker acid HCl, by what I call a reverse double displacement reaction.
Making H2SO4 from Sulfates and HCl
Make Copper Sulfate from Copper and Sulfuric acid (3 ways)
How to Make Sulfuric Acid
Make Sulfuric Acid (Copper Sulfate Electrochemical Method)
Making Sulfuric Acid From Epsom Salt
How to Make Sulfuric Acid (Copper Sulfate)
How to Make Sulfuric Acid From Copper Sulfate And Sulfur Dioxide at Home
Make Sulfuric acid (metabisulfite/oxidizer method)
Making Salts - GCSE Science Required Practical
Preparation of Sulphuric Acid (H2SO4) With Electrolysis of Copper Sulphate (CuSO4)
sulphuric acid #shorts
NaCl and H2SO4 #shorts #chemistryexperiments #shortsfeed
Sulfuric Acid on Toilet Paper Spawns a Demon
potassium iodide and sulphuric acid #shorts #experiment #shortsviral
How To Make Sodium Sulfate (NaHSO4) At Home
How to make Sulfur
barium chloride and sodium sulphate 10th activity #shorts #scienceexperiment
GCSE Chemistry Revision 'Required Practical 1: Making Soluble Salts'
Making of Sulphuric Acid H2SO4 | Sulphuric Acid Formula | Labkafe
match boxes and sulphuric acid #chemistry #shorts #shortsfeed
chemistry science experiment -sugar & h2so4 #shorts #labxyz #chemistryscience
Making Copper Sulphate from Copper Oxide
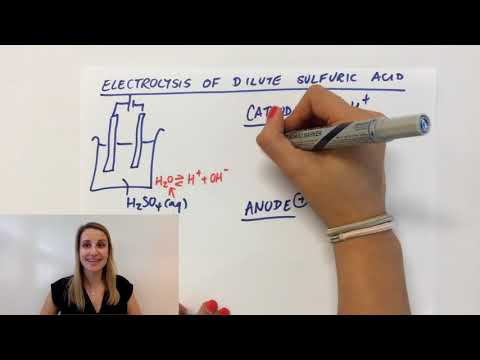
Explaining the electrolysis of dilute sulfuric acid H2SO4 (aq) GCSE Chemistry
How to make sulfuric acid
Комментарии
 0:04:39
0:04:39
 0:04:01
0:04:01
 0:03:44
0:03:44
 0:04:08
0:04:08
 0:19:18
0:19:18
 0:10:39
0:10:39
 0:06:26
0:06:26
 0:03:43
0:03:43
 0:05:36
0:05:36
 0:04:48
0:04:48
 0:00:17
0:00:17
 0:00:22
0:00:22
 0:00:38
0:00:38
 0:00:23
0:00:23
 0:04:50
0:04:50
 0:00:18
0:00:18
 0:00:14
0:00:14
 0:04:51
0:04:51
 0:00:31
0:00:31
 0:00:21
0:00:21
 0:00:46
0:00:46
 0:02:06
0:02:06
 0:03:01
0:03:01
 0:00:50
0:00:50