filmov
tv
Why does like dissolve like?

Показать описание
Talking about why polar substances mix together i.e. polar solutes dissolve in polar solvents, nonpolar substances mix together i.e. nonpolar solutes dissolve in nonpolar solvents, but a polar substance and a nonpolar substance do not mix together to form a solution.
😀 Thanks for watching! 😀
SUPPORT THE STREAM:
🔴 Crypto:
👉 Bitcoin: 35Sp5MATXBTpCgNs8pHcHYENPZ6Rd2Mh5A
👉 Etherium: 0x07Ba1EB379C943bA5Ce2C1c2939Cbe64637f0B06
👉 Litecoin: MWaksUUAsPG71hVTc9q7EmK1aDzqpRjbnJ
Follow me on:
🎤 Song: Lucid, by Smith the Mister
#Chemistry #Solution #Polarity #Solutions #Dissolve #LikeDissolvesLike #Polar #Solute #Solvent #Entropy #Energy #IntermolecularForces #Dipole #HydrogenBonding #HydrogenBond
😀 Thanks for watching! 😀
SUPPORT THE STREAM:
🔴 Crypto:
👉 Bitcoin: 35Sp5MATXBTpCgNs8pHcHYENPZ6Rd2Mh5A
👉 Etherium: 0x07Ba1EB379C943bA5Ce2C1c2939Cbe64637f0B06
👉 Litecoin: MWaksUUAsPG71hVTc9q7EmK1aDzqpRjbnJ
Follow me on:
🎤 Song: Lucid, by Smith the Mister
#Chemistry #Solution #Polarity #Solutions #Dissolve #LikeDissolvesLike #Polar #Solute #Solvent #Entropy #Energy #IntermolecularForces #Dipole #HydrogenBonding #HydrogenBond
Why does like dissolve like?
How Solubility and Dissolving Work
Solubility and intermolecular forces | AP Chemistry | Khan Academy
Polar & Non-Polar Molecules: Crash Course Chemistry #23
38: Using 'like dissolves like' to predict solubility
Like dissolves Like Lecture
#17 like dissolves like
ALEKS: Applying like dissolves like
'Like dissolves like' rule in Chemistry.What does the statement describes? Solubility of s...
Polar and Non polar. Like dissolves like . Solubility physical pharmaceutics semester 3
Solubility of Organic Compounds
Chem 117 Week 5 Lecture 1B Like Dissolves Like
Principle of Like Dissolves Like |Solubility and its Factors|
Solutions: Why Do Things Dissolve? Polar, Non-Polar and Ionic Compounds
Like dissolves like
'Like Dissolves Like' Demo
Like Dissolves Like
IMF and Solubility
Aleks Applying like dissolves like
30 LIKE DISSOLVE LIKE RULE
What is the Difference Between Polar and Non - Polar Substances | Chemistry Concepts
'Like Dissolves Like' Demo - to dissolve CuSO4
Polarity and Dissolving in Chemistry
Like Dissolve Like - Experiment
Комментарии
 0:08:01
0:08:01
 0:04:29
0:04:29
 0:04:23
0:04:23
 0:10:46
0:10:46
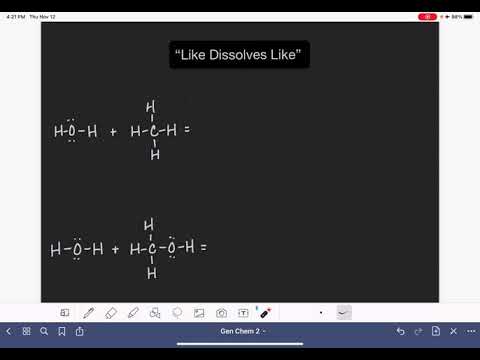 0:03:41
0:03:41
 0:05:39
0:05:39
 0:01:23
0:01:23
 0:09:11
0:09:11
 0:04:58
0:04:58
 0:03:38
0:03:38
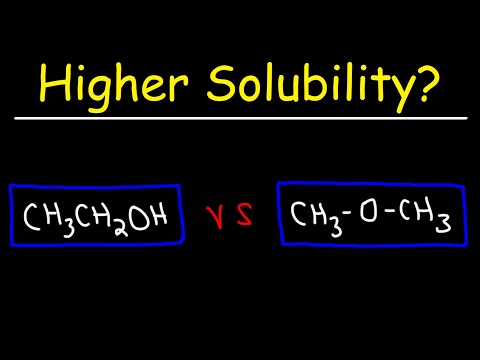 0:10:36
0:10:36
 0:08:06
0:08:06
 0:05:46
0:05:46
 0:11:55
0:11:55
 0:03:45
0:03:45
 0:02:19
0:02:19
 0:15:07
0:15:07
 0:06:57
0:06:57
 0:07:12
0:07:12
 0:07:55
0:07:55
 0:02:19
0:02:19
 0:02:15
0:02:15
 0:13:06
0:13:06
 0:02:58
0:02:58