filmov
tv
Geometrical isomerism - Cis - Trans Isomerism - Explained shortly
Показать описание
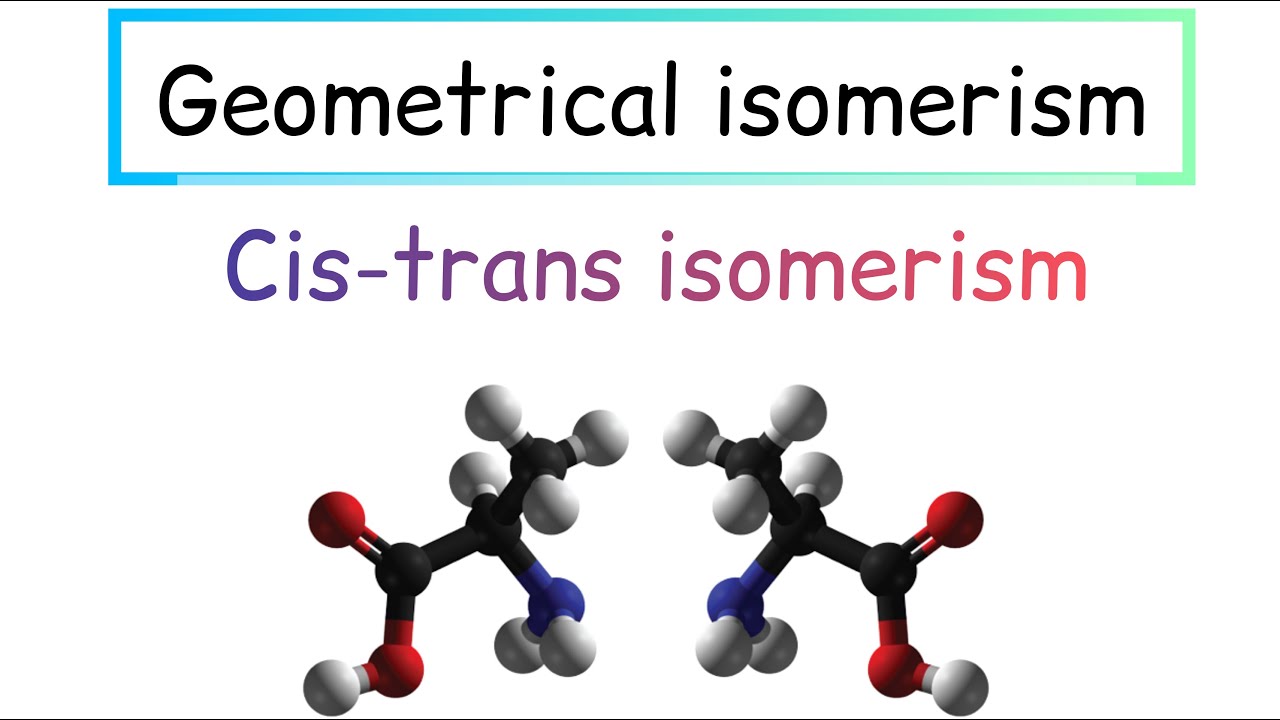
Geometrical isomerism
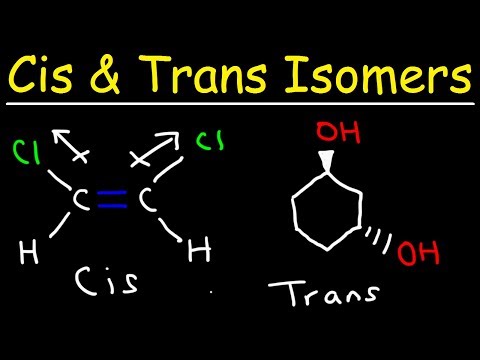
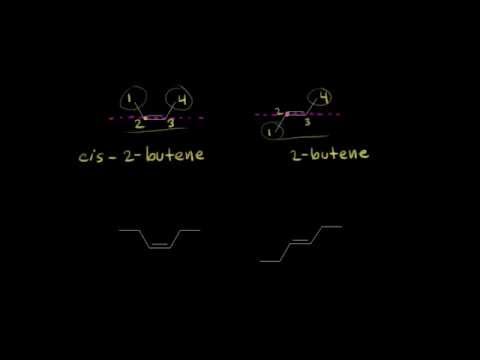
The particular kind of diastereomers, that owe their existence, to hindered rotation about double bonds, are called geometric isomers.
Conditions for a compound to exhibit geometrical isomerism:
1. The compound must contain double bonded system.
2. To each doubly bonded atom there should be, two unlike atoms or groups.
0:00 Introduction
0:50 Definition of geometrical isomerism
1:06 Conditions for a compound to exhibit geometrical isomerism
1:49 Examples
#Cis #Trans #Isomerism #Geometric #Isomer #Diasteromers #Diasteromerism #Chemistry #Organic #Atoms #Molecules #Concepts #propylene #Butene #Aldoxime #Syn #Anti #2-Butene #Chemistry #GPAT #NIPER #Pharmacy #Pharma #Pharmaceutical
The particular kind of diastereomers, that owe their existence, to hindered rotation about double bonds, are called geometric isomers.
Conditions for a compound to exhibit geometrical isomerism:
1. The compound must contain double bonded system.
2. To each doubly bonded atom there should be, two unlike atoms or groups.
0:00 Introduction
0:50 Definition of geometrical isomerism
1:06 Conditions for a compound to exhibit geometrical isomerism
1:49 Examples
#Cis #Trans #Isomerism #Geometric #Isomer #Diasteromers #Diasteromerism #Chemistry #Organic #Atoms #Molecules #Concepts #propylene #Butene #Aldoxime #Syn #Anti #2-Butene #Chemistry #GPAT #NIPER #Pharmacy #Pharma #Pharmaceutical
 0:06:35
0:06:35
 0:05:04
0:05:04
 0:15:21
0:15:21
 0:05:06
0:05:06
 0:05:24
0:05:24
 0:02:41
0:02:41
 0:03:24
0:03:24
 0:20:23
0:20:23
 0:40:40
0:40:40
 0:11:15
0:11:15
 0:09:17
0:09:17
 0:00:13
0:00:13
 0:00:35
0:00:35
 0:09:26
0:09:26
 0:00:19
0:00:19
 0:08:25
0:08:25
 0:06:01
0:06:01
 0:00:54
0:00:54
 0:00:39
0:00:39
 0:20:59
0:20:59
 0:00:16
0:00:16
 0:00:39
0:00:39
 0:00:43
0:00:43
 0:00:54
0:00:54