filmov
tv
How to Write Ionic Half Equations in Electrolysis Questions (GCSE Chemistry)

Показать описание
In this video I am going to teach you how to write ionic half equations which are really useful in understanding electrolysis. We will also look at OIL RIG and how to identify whether an ion is being oxidised or reduced.
It actually turns out that writing ionic half equations is really as easy as 1,2,3
1. The ions turn into atoms
2. Balance the symbols
3. Balance the charges.
It actually turns out that writing ionic half equations is really as easy as 1,2,3
1. The ions turn into atoms
2. Balance the symbols
3. Balance the charges.
How to Write Ionic Half Equations in Electrolysis Questions (GCSE Chemistry)
Ionic Equations - GCSE Chemistry Revision
Ionic Equation
How To Write Net Ionic Equations In Chemistry - A Simple Method!
What Are Half Equations | Reactions | Chemistry | FuseSchool
Half Reaction Method, Balancing Redox Reactions In Basic & Acidic Solution, Chemistry
GCSE Chemistry - Oxidation and Reduction - Redox Reactions #39 (Higher Tier)
Ionic half equations
Chemistry AMS Explained by my friend
A level Chemistry: Step by Step guide on HOW to write IONIC Equations PERFECTLY every single time
Writing half-reactions (ionic equations and net ionic equations)
IONIC EQUATIONS: The explanation behind how to solve + a shortcut!
HALF EQUATIONS | IONIC EQUATIONS | With examples on how to balance half equations | SPM
Ionic Equations | Reactions | Chemistry | FuseSchool
GCSE Chemistry - Balancing Chemical Equations #4
Ionic Half Equations EASY Explanation - GCSE & IGCSE Chemistry Revision 2024
O level Chemistry - How to write ionic equation
Introduction to Oxidation Reduction (Redox) Reactions
Balancing Redox Reactions in Acidic and Basic Conditions
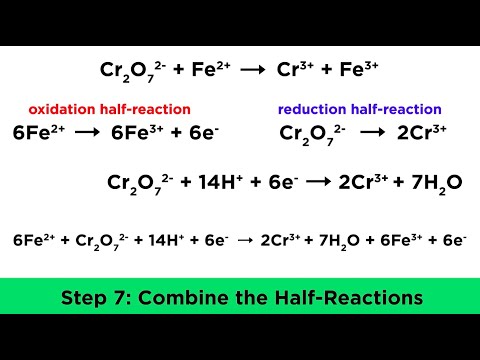
Balancing ionic half equations for redox reactions | A-Level Chemistry
How to Write and Balance Net Ionic Equations
How to Write Complete Ionic Equations and Net Ionic Equations
Chemistry 13.4 Writing Half-reactions for Redox
Balance redox reaction (ionic half equation method)
Комментарии
 0:05:58
0:05:58
 0:03:21
0:03:21
 0:02:58
0:02:58
 0:10:48
0:10:48
 0:03:53
0:03:53
 0:16:00
0:16:00
 0:04:54
0:04:54
 0:04:44
0:04:44
 0:26:21
0:26:21
 0:13:05
0:13:05
 0:03:56
0:03:56
 0:06:27
0:06:27
 0:12:52
0:12:52
 0:04:32
0:04:32
 0:05:18
0:05:18
 0:06:25
0:06:25
 0:11:17
0:11:17
 0:13:05
0:13:05
 0:07:31
0:07:31
 0:03:42
0:03:42
 0:06:18
0:06:18
 0:09:03
0:09:03
 0:07:17
0:07:17
 0:04:29
0:04:29