filmov
tv
Is OF2 Polar or Nonpolar? (Oxygen Difluoride)

Показать описание
Hey Guys!
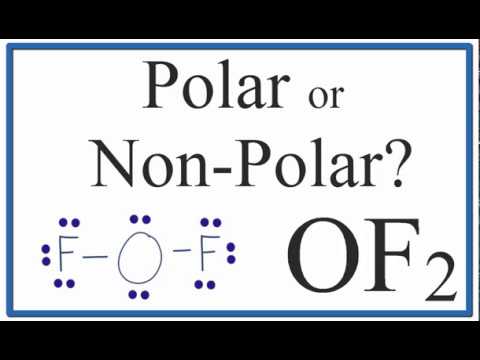
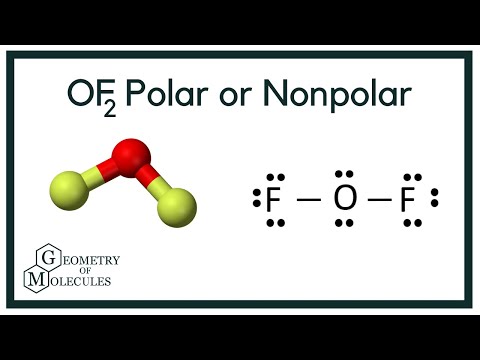
In this video, we are going to determine the polarity of Oxygen Difluoride having a chemical formula of OF2. It is made of one Oxygen atom and two Fluorine atoms.
To know the polarity of this molecule we will first look at the Lewis Structure of Oxygen Difluoride followed by its shape. The Lewis Structure of the molecule helps to determine the net dipole moment in the molecule.
► Below are the Tools we use to make our Videos more engaging :
Thanks for watching !!
#OF2Polarity #OxygenDifluoridePolarity #OxygenDifluoride
In this video, we are going to determine the polarity of Oxygen Difluoride having a chemical formula of OF2. It is made of one Oxygen atom and two Fluorine atoms.
To know the polarity of this molecule we will first look at the Lewis Structure of Oxygen Difluoride followed by its shape. The Lewis Structure of the molecule helps to determine the net dipole moment in the molecule.
► Below are the Tools we use to make our Videos more engaging :
Thanks for watching !!
#OF2Polarity #OxygenDifluoridePolarity #OxygenDifluoride
Is OF2 Polar or Nonpolar? (Oxygen Difluoride)
Is OF2 Polar or Nonpolar? (Oxygen Difluoride)
Is OF2 Polar or Nonpolar? (Oxygen Difluoride)
Is Nitric oxide (NO) Polar or Non-Polar?
Is SF2 Polar or Nonpolar? (Sulfur Difluoride)
Is F2 Polar or Non-polar? (Fluorine Gas)
Is SiCl4 Polar or Non-Polar?
Is N2O polar or nonpolar?
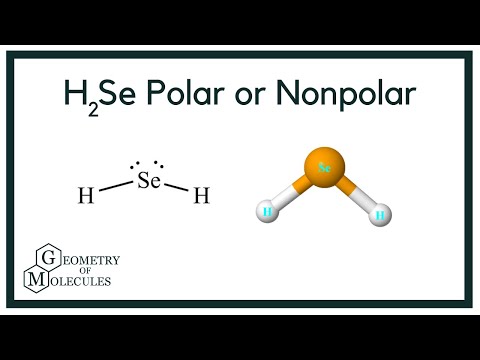
Is H2Se Polar or Nonpolar? (Hydrogen Selenide)
s BeCl2 Polar or Nonpolar beryllium chloride
Is NOCl Polar or Non-Polar (Nitrosyl chloride)
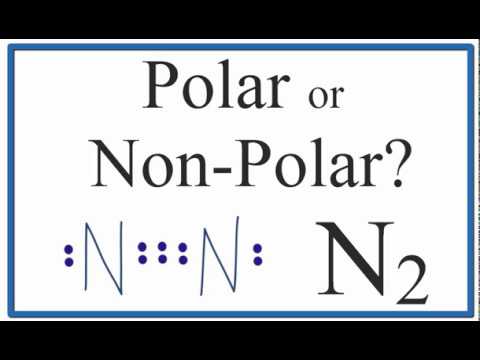
Is N2 Polar or Non-polar? (Nitrogen Gas)
Is SiH4 Polar or Nonpolar? (Silicon Tetrahydride)
Is IF2- polar or nonpolar?
Is CH3F (Fluoromethane) Polar or Non-Polar?
Is CS2 polar or nonpolar? (Carbon Disulfide)
Is CH2O Polar or Nonpolar? (Methanal or Formaldehyde)
Polar or Nonpolar? Electron Domain Geometries of 2, 3, & 4
Is CH3OH Polar or Nonpolar? (Methanol)
Is NOCl polar or nonpolar?
sf4 polar or nonpolar
Is CHF3 Polar or Nonpolar?
Is CF2Cl2 Polar or Non-Polar?
Is SCl2 polar or nonpolar?
Комментарии
 0:02:18
0:02:18
 0:01:42
0:01:42
 0:01:42
0:01:42
 0:01:11
0:01:11
 0:01:24
0:01:24
 0:01:11
0:01:11
 0:01:51
0:01:51
 0:03:24
0:03:24
 0:01:39
0:01:39
 0:01:59
0:01:59
 0:01:47
0:01:47
 0:01:11
0:01:11
 0:01:29
0:01:29
 0:11:04
0:11:04
 0:01:33
0:01:33
 0:01:14
0:01:14
 0:01:38
0:01:38
 0:10:14
0:10:14
 0:01:21
0:01:21
 0:04:06
0:04:06
 0:02:28
0:02:28
 0:01:34
0:01:34
 0:02:11
0:02:11
 0:09:12
0:09:12