filmov
tv
Lecture 13: Ideal Gases: Internal Energy, Enthalpy, and Specific Heats (Engineering Thermodynamics)

Показать описание
In this lecture, we apply the first law of thermodynamics to ideal gases that obey pv=RT. We show that such gases have internal energy and enthalpy that are functions of temperature only. We also derive the relation between the specific heats at constant pressure and constant volume for an ideal gas. Finally, we apply the first law to calculate the work done during quasi-static, adiabatic and isothermal change in volume of an ideal gas. (c) Supreet Singh Bahga
Lecture 13: Ideal Gases: Internal Energy, Enthalpy, and Specific Heats (Engineering Thermodynamics)
Lecture 13: Introduction to Ideal (Gas) Mixtures
Chem 312 Lecture 13 Ideal Gases 1 4-9-20
Thermodynamics and Fluid Mechanics | C2 - L13 | Internal Energy of Ideal Gas
The Ideal Gas Law: Crash Course Chemistry #12
Thermodynamics and Chemical Dynamics 131C. Lecture 05. The Equipartition Theorum.
Thermodynamics L3 Internal Energy, Enthalpy and Specific Heats
Lecture 13 - MECH 2311 - Introduction to Thermal Fluid Science
Lecture 7: Ideal Gas Processes
Lecture 13 Chapter 13 Gas mixtures
Thermodynamics and Chemical Dynamics 131C. Lecture 13. The Carnot Cycle.
Lecture 16.2 Ideal Gas Law
University Physics Lectures, Molecular Model of an Ideal Gas
Universty Physics Lectures, Macroscopic Description of an Ideal Gas
Thermodynamics by Yunus Cengel - Lecture 13: 'Chap 4: Solid, liquid, ideal gas energy analysis&...
Physics 9B - Lecture 13
Ideal Gas Law Practice Problems
Internal Energy | Enthalpy | Specific Heat | Ideal gases | Temperatures | Heat Transfer Modes
Exact Calculation of the Internal Energy for Ideal Gas in Statistical Mechanics
T37 Internal energy enthalpy and specific heats of ideal gases [S4.4 in Arabic]
Introduction to Physics (Aleksey Ilyin). Lecture 13
Ideal Gas Law & Ideal Gas Equation [Practice Problem Solved!]
ECHE 310 - Lecture 13 - Applying the 2nd Law: Reversibility and Feasibility of Chemical Processes
Statistical Thermodynamics. Lecture 13. Thermodynamic properties of monoatomic gas
Комментарии
 0:36:03
0:36:03
 0:49:57
0:49:57
 0:29:41
0:29:41
 0:07:46
0:07:46
 0:09:03
0:09:03
 0:51:06
0:51:06
 0:11:26
0:11:26
 0:08:46
0:08:46
 0:46:23
0:46:23
 0:30:32
0:30:32
 0:46:12
0:46:12
 0:12:24
0:12:24
 0:12:22
0:12:22
 0:10:24
0:10:24
 0:43:57
0:43:57
 1:32:45
1:32:45
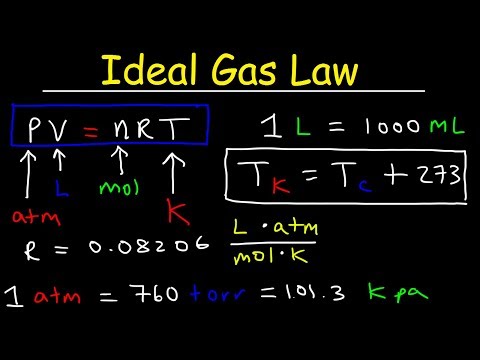 0:12:27
0:12:27
 0:42:02
0:42:02
 0:01:10
0:01:10
 0:11:24
0:11:24
 1:28:31
1:28:31
 0:07:31
0:07:31
 0:36:56
0:36:56
 0:22:18
0:22:18