filmov
tv
16.1/R2.2.6 Reaction mechanism, order of reaction and rate-determining step [HL IB Chemistry]

Показать описание
Rate = k [product of the reactants in the rate determining step] but it could be more complex - see the vid.
Make sure that when you "add up" the mechanism it equals your initial given equation.
Was the stair that Dr Atkinson demised on his personal "rate determining step"?
Make sure that when you "add up" the mechanism it equals your initial given equation.
Was the stair that Dr Atkinson demised on his personal "rate determining step"?
Writing Rate Laws of Reaction Mechanisms Using The Rate Determining Step - Chemical Kinetics
IB Chemistry Topic 6 Kinetics 16.1 Rate expression and reaction mechanism
R2.2.10 Orders of reaction (HL)
IB Chemistry Topic 16.1 & 16.2 (HL) - Part 2: Reaction Mechanisms and Activation Energy
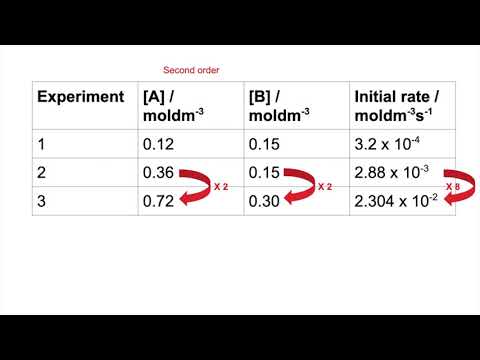
Working out order from a rate table - tricky example
Most💯 Important Step Before any Procedure 🔥
IB Chem Topic 6/16 Kinetics Lesson 4 Reaction Mechanisms
16.1/R2.2.9 Solve problems involving the rate expression [HL IB Chemistry]
Chapter 03 | Chemical Kinetics | Lecture 6 | Order of reaction from Mechanism of Complex Reaction.
Chemistry 202. Organic Reaction Mechanisms II. Lecture 16. Kinetics and Rate Equations
Kinetics IB Questions - Answers Explained
A case that shocked Canada in 2012😳 #shorts
His reaction when he sees her FEET for the first time…😳 #Shorts
Sodium metal, soft, reactive, and squishy
India's first hoverboard 😨
Tough times Never last 😊✌️ #delhipolice #motivation
1st yr. Vs Final yr. MBBS student 🔥🤯#shorts #neet
How much does a PHYSICS RESEARCHER make?
⚠️*TRUSTS TESLA AUTOPILOT* ⚠️ IMMEDIATE REGRET 😳🛑 ALMOST CRASHES ⚠️ WOULD YOU TRUST THIS⁉️ #Shorts...
Transvaginal Test For Females #shorts
Aspirants practicing eatingetiquette # SSB #SSBPreparation #NDA #CDS #Defence #DefenceAcademy
asking minor test marks to allen topper allen kota #allen #allenkota #physicswallah #pw
SN1 SN2 E1 E2 Reaction Mechanism - Test Review
Salsa Night in IIT Bombay #shorts #salsa #dance #iit #iitbombay #motivation #trending #viral #jee
Комментарии
 0:18:48
0:18:48
 0:14:03
0:14:03
 0:06:15
0:06:15
 0:04:56
0:04:56
 0:02:46
0:02:46
 0:00:16
0:00:16
 0:19:42
0:19:42
 0:04:16
0:04:16
 0:21:00
0:21:00
 0:50:58
0:50:58
 0:26:51
0:26:51
 0:00:14
0:00:14
 0:01:00
0:01:00
 0:00:50
0:00:50
 0:00:26
0:00:26
 0:00:22
0:00:22
 0:00:20
0:00:20
 0:00:44
0:00:44
 0:00:23
0:00:23
 0:00:24
0:00:24
 0:00:11
0:00:11
 0:00:30
0:00:30
 0:59:10
0:59:10
 0:00:14
0:00:14