filmov
tv
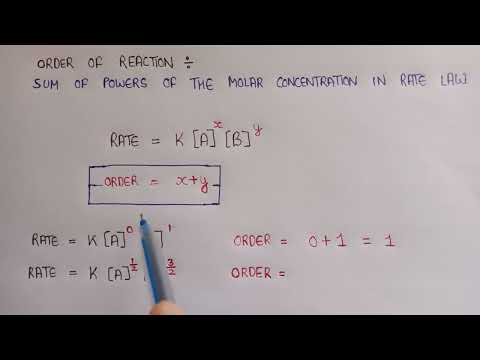
Determine Order of Reaction from Initial Rates

Показать описание
Comparing initial rates to determine the order of reaction is a very common question in Kinetics.
Let's take a look at an example:
The objective is to choose a pair of experiments for comparison where the concentration of a reactant changes and ideally the concentration of other reactants remain constant.
This means that any change in the initial rates of the experiments must be due to the change in the concentration of that reactant, and we can figure out the order from there.
1. Order of Reaction with respect to HCl
Comparing experiments 1 and 3, concentration of HCl doubles and there is no change in concentration of sucrose.
So the change in initial rates must be due to HCl only.
We can work out the change in initial rates to be 2 times.
This means when concentration of HCl doubles, initial rate doubles.
Therefore order of the reaction with respect to HCl will be order 1.
For comparison, if order of reaction is zero, initial rate will remain unchanged when concentration of HCl doubles.
If order of reaction is 2, initial rate will increase by 4 times (2^2 times) when concentration of HCl doubles.
Since we only have 3 possible orders to consider, figuring out the order of reaction is quite straightforward.
2. Order of Reaction with respect to sucrose
Comparing experiments 1 and 2, concentration of sucrose increase by 1.5 times and there is no change in concentration of HCl.
So the increase in initial rates by 1.5 times must be due to sucrose only.
Since this is a proportionate increase, order of reaction with respect to sucrose is also order 1.
Finally we can write out the rate equation for this reaction to be:
rate = k [HCl][sucrose]
Topic: Kinetics, Physical Chemistry, A Level Chemistry, Singapore
Found this video useful?
Please LIKE this video and SHARE it with your friends!
Any feedback, comments or questions to clarify?
Suggestions for new video lessons?
Drop them in the COMMENTS Section, I would love to hear from you!
You can also view this video lesson with screenshots and detailed explanation at
Do check out the following for more video lessons:
-~-~~-~~~-~~-~-
Please watch my latest video: "2019 P1 Q2 - Deflection of Charged Particle in Electric Field"
-~-~~-~~~-~~-~-
Let's take a look at an example:
The objective is to choose a pair of experiments for comparison where the concentration of a reactant changes and ideally the concentration of other reactants remain constant.
This means that any change in the initial rates of the experiments must be due to the change in the concentration of that reactant, and we can figure out the order from there.
1. Order of Reaction with respect to HCl
Comparing experiments 1 and 3, concentration of HCl doubles and there is no change in concentration of sucrose.
So the change in initial rates must be due to HCl only.
We can work out the change in initial rates to be 2 times.
This means when concentration of HCl doubles, initial rate doubles.
Therefore order of the reaction with respect to HCl will be order 1.
For comparison, if order of reaction is zero, initial rate will remain unchanged when concentration of HCl doubles.
If order of reaction is 2, initial rate will increase by 4 times (2^2 times) when concentration of HCl doubles.
Since we only have 3 possible orders to consider, figuring out the order of reaction is quite straightforward.
2. Order of Reaction with respect to sucrose
Comparing experiments 1 and 2, concentration of sucrose increase by 1.5 times and there is no change in concentration of HCl.
So the increase in initial rates by 1.5 times must be due to sucrose only.
Since this is a proportionate increase, order of reaction with respect to sucrose is also order 1.
Finally we can write out the rate equation for this reaction to be:
rate = k [HCl][sucrose]
Topic: Kinetics, Physical Chemistry, A Level Chemistry, Singapore
Found this video useful?
Please LIKE this video and SHARE it with your friends!
Any feedback, comments or questions to clarify?
Suggestions for new video lessons?
Drop them in the COMMENTS Section, I would love to hear from you!
You can also view this video lesson with screenshots and detailed explanation at
Do check out the following for more video lessons:
-~-~~-~~~-~~-~-
Please watch my latest video: "2019 P1 Q2 - Deflection of Charged Particle in Electric Field"
-~-~~-~~~-~~-~-
Комментарии
 0:01:58
0:01:58
 0:05:45
0:05:45
 0:18:48
0:18:48
 0:03:50
0:03:50
 0:10:20
0:10:20
 0:10:39
0:10:39
 0:01:33
0:01:33
 0:06:15
0:06:15
 0:44:29
0:44:29
 0:03:03
0:03:03
 0:09:29
0:09:29
 0:08:20
0:08:20
 0:05:09
0:05:09
 0:36:30
0:36:30
 0:09:02
0:09:02
 0:09:21
0:09:21
 0:05:12
0:05:12
 0:08:06
0:08:06
 0:16:18
0:16:18
 0:02:42
0:02:42
 0:06:14
0:06:14
 0:05:09
0:05:09
 0:07:46
0:07:46
 0:12:21
0:12:21