filmov
tv
Best Trick of Electronic configuration in KLMN /by ramgeet academy.

Показать описание
Hello friends maine is video me klmn me electronic configuration karna bataya hau ak trick ke basis par.
I hope that you enjoyed this video....
How to write the electron configuration of an ion, How to write orbital notation with arrows, How to write orbital diagrams, Electronic structure, Shell:orbital diagrams and electron configuration, Electron diagrams, Electron configuration, How do you draw an electron configuration diagram, Electron configuration (literature subject), Electron configuration:khan academy, Periodic table (invention), Electron (subatomic particle), Chemistry (field of study), Quantum mechanics (field of study),Subshells:electron configuration (literature subject),Subshells and orbitals explained,Electronic configuration of d block elements,Electronic configuration of s block elements,Practice problems,Electronic configuration,How to write the electron configuration,How to find the electron configuration
Blogger link
Thank you for watching #RamGeetAcademy
#ankitnishad #ankit #ramgeet #study
#electroniccnfiguration #klmn #electron #priton #neutron #electronic #configuration
I hope that you enjoyed this video....
How to write the electron configuration of an ion, How to write orbital notation with arrows, How to write orbital diagrams, Electronic structure, Shell:orbital diagrams and electron configuration, Electron diagrams, Electron configuration, How do you draw an electron configuration diagram, Electron configuration (literature subject), Electron configuration:khan academy, Periodic table (invention), Electron (subatomic particle), Chemistry (field of study), Quantum mechanics (field of study),Subshells:electron configuration (literature subject),Subshells and orbitals explained,Electronic configuration of d block elements,Electronic configuration of s block elements,Practice problems,Electronic configuration,How to write the electron configuration,How to find the electron configuration
Blogger link
Thank you for watching #RamGeetAcademy
#ankitnishad #ankit #ramgeet #study
#electroniccnfiguration #klmn #electron #priton #neutron #electronic #configuration
Комментарии
 0:04:36
0:04:36
 0:10:19
0:10:19
 0:00:50
0:00:50
 0:07:23
0:07:23
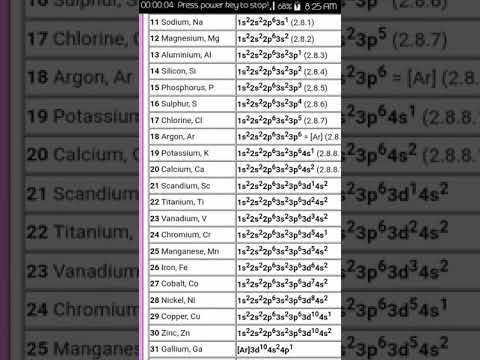 0:00:12
0:00:12
 0:07:48
0:07:48
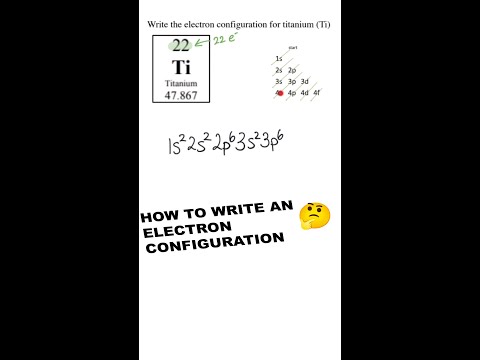 0:01:00
0:01:00
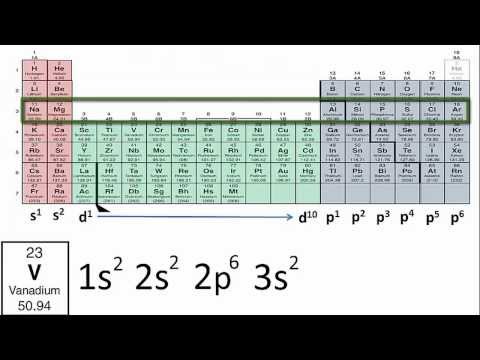 0:04:52
0:04:52
 0:02:01
0:02:01
 0:08:42
0:08:42
 0:00:56
0:00:56
 0:18:56
0:18:56
 0:13:36
0:13:36
 0:04:59
0:04:59
 0:17:04
0:17:04
 0:00:57
0:00:57
 0:00:32
0:00:32
 0:01:00
0:01:00
 0:00:56
0:00:56
 0:23:12
0:23:12
 0:12:57
0:12:57
 0:09:05
0:09:05
 0:05:26
0:05:26
 0:01:00
0:01:00