filmov
tv
Exam 3 Review

Показать описание
Exam 3 Review Health Assessment
how to STUDY FOR AN EXAM in 3 days and SCORE A+ (exam hacks you didn't know)
Physics Exam 3 Review
How to Finish 6 Months of Study in 72 Hours
Statics: Exam 3 Review Summary
Statics: Exam 3 Review Problem 2; Frame Example
Statics: Exam 3 Review Problem 4, Shear Moment Diagram Example
Process Control Exam 3 Review
Statics: Exam 3 Review Problem 5, Simple Friction is Fun
how to study SMART for EXAMS | test/exam tips
Statics: Exam 3 Review Problem 6, Friction Slipping Tipping
Statics: Exam 3 Review Problem 1; Truss Combo Method
Search quality rater exam phase 3 | welocalize client exam 3
How to CRAM for an Exam in 24 Hours
Exam 3 Review
Last Minute Exam Tips to SAVE Your Grades | How I Crammed in 5 Days in 5 Steps
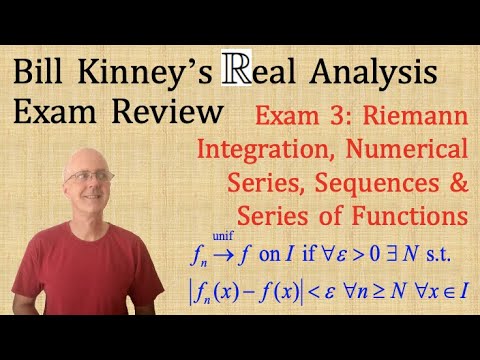
Real Analysis Exam 3 Review Problems and Solutions
3-day Study Plan! // How to prepare for any exam in 72 hours
Calculus I Test 3 Review
how to STUDY for an exam THE NIGHT BEFORE (and still get all As)
Exam Assistance: Telus Assessor/ Rater Exam Welocalize Search Rater, RWS Moravia Rater Exam Part 3
how to EFFECTIVELY STUDY the night before a test
Multivariable Calculus Final Exam Review
how to CRAM for an exam (the right way)
Комментарии
 0:48:59
0:48:59
 0:08:21
0:08:21
 0:25:35
0:25:35
 0:18:28
0:18:28
 0:06:50
0:06:50
 0:12:41
0:12:41
 0:22:29
0:22:29
 0:50:57
0:50:57
 0:16:32
0:16:32
 0:08:18
0:08:18
 0:17:57
0:17:57
 0:18:36
0:18:36
 0:02:40
0:02:40
 0:08:43
0:08:43
 1:57:51
1:57:51
 0:13:15
0:13:15
 1:35:00
1:35:00
 0:06:53
0:06:53
 1:45:49
1:45:49
 0:05:35
0:05:35
 0:06:19
0:06:19
 0:04:13
0:04:13
 1:17:35
1:17:35
 0:07:29
0:07:29